Polyolefins, a versatile class of polymers originating from simple olefins (alkenes), are characterized by their low density, exceptional chemical resistance, and zero moisture absorption. These inherent qualities enable their widespread use in diverse applications, ranging from thin films to robust natural gas pipelines. This article delves into the world of polyolefins, providing a comprehensive look at their key properties, processing techniques, and common applications.
What are Polyolefins?
Polyolefins are hydrocarbon-based materials characterized by their repeating units of carbon and hydrogen, leading to a wide range of properties depending on the specific olefin monomer used and the polymerization process. Polyolefins are some of the most widely produced plastics globally.
Types of Polyolefins
Polyethylene (PE)
Polyethylene (PE), the foundational member of the polyolefin family, exhibits a broad spectrum of grades and structural arrangements, including the well-known low-density (LDPE) and high-density (HDPE) variants. This variety in structure leads to a wide array of properties, largely dictated by the degree of molecular branching (which influences density, Figure 1) and the length of its molecular chains (molecular weight, shown in Figure 2).
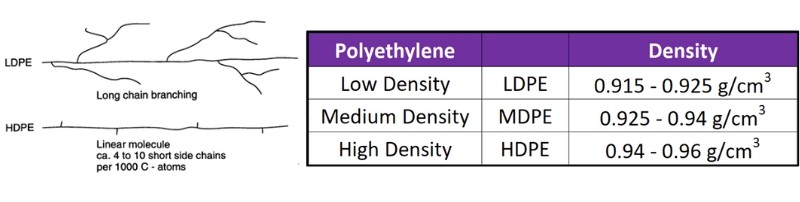
Figure 1. Branching of polyethylene’s effect on density.

Figure 2. Polyethylene crystallinity versus molecular weight.
Key characteristics of polyethylene include the following:
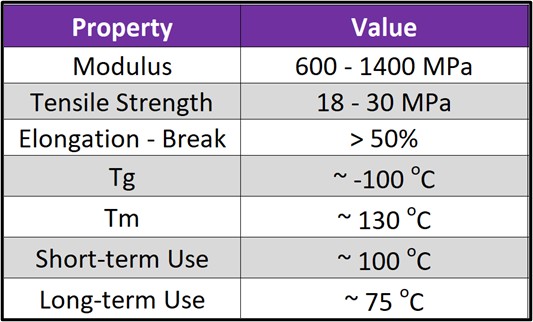
Figure 3. Typical properties of polyethylene.
- Low Density: Unfilled grades of PE will float.
- Ease of Processing: Nonpolar nature typically does not require drying.
- Low Cost: Combined with low density, it offers a low-cost part.
- High Elongation at Break: A tough material, though relative strength is low, Figure 3.
- Excellent Cold Temperature Properties: Very low glass transition temperature (Tg) ~ -100 °C, suitable for refrigerated goods.
- Low Water Absorption: Very nonpolar – attractive property for containers, packaging, piping.
- High Chemical & ESC Resistance: Highly crystalline polymer.
Despite its many advantageous properties, PE presents certain challenges. It’s very low oxidation resistance necessitates the use of antioxidants during both processing and its service life. Furthermore, achieving strong adhesive bonds with PE can be difficult, making hot plate welding the prevalent joining technique. PE also exhibits low creep resistance, a consequence of its low Tg. Finally, its high flammability, essentially behaving as a solid fuel, often requires the incorporation of flame retardants for specific applications.
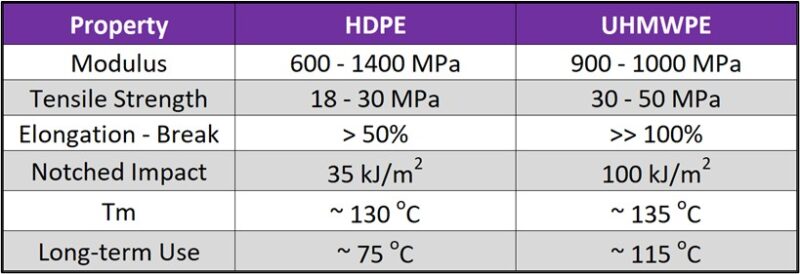
Figure 4. Typical properties of HDPE versus UHMWPE.
While standard polyethylene can exist in branched or linear forms, UHMWPE (Ultra-High Molecular Weight Polyethylene) is a linear polymer with very minimal branching. The key characteristic of UHMWPE is its extremely long polymer chains, resulting in a molecular weight ranging from 3.1 to 100 million g/mol, which are significantly longer than those in HDPE or LDPE. These long chains align themselves in the same direction and have strong intermolecular interactions (Van der Waals forces along the extended chain). The linear structure of UHMWPE is fundamental to its exceptional performance characteristics, notably its high abrasion resistance, low coefficient of friction, and impressive impact strength. Indeed, UHMWPE stands out as an extremely tough material, boasting one of the highest impact strengths among all thermoplastics. Beyond its mechanical robustness, it offers superior chemical resistance compared to standard polyethylene, Figure 4, and a remarkably low coefficient of friction, making it a potential substitute for PTFE in various applications. This inherent abrasion resistance further enhances its suitability for demanding environments and applications where wear is a primary concern. It is important to note that UHMWPE is difficult to process, and parts are often machined from stock shapes.
Polypropylene (PP)
Polypropylene (PP) is another significant polyolefin, closely related to polyethylene but with a key structural difference: the presence of a methyl group (-CH3) attached to every other carbon atom in the polymer backbone. This seemingly small change leads to notable differences in its properties compared to PE.
While both are versatile and widely used polyolefins, polypropylene is stiffer, harder, has a higher melting point and better chemical resistance at room temperature, and is less dense than most polyethylene grades. Polyethylene, on the other hand, often offers better flexibility and impact resistance at low temperatures.
Key characteristics of polypropylene include the following:
- Low Density: One of the lightest polymers.
- Ease of Processing: Nonpolar – typically doesn’t require drying.
- Low Cost: Combined with low density, it leads to a low-cost part.
- High Elongation at Break: A tough material, though relative strength is low.
- Low Water Absorption: Very nonpolar.
- High Chemical & ESC Resistance: Highly crystalline polymer.
- Copolymers: Properties can be tailored.
Despite its beneficial attributes, PP also faces several limitations. Its cold temperature performance is poor, stemming from a Tg typically ranging between -5 and +15 °C. Similar to polyethylene, PP exhibits very low oxidation resistance, necessitating the inclusion of antioxidants during both manufacturing and its service life. Achieving strong adhesive bonds with PP is also challenging, making hot plate welding the generally preferred method for joining components. Furthermore, PP demonstrates low creep resistance, especially since room temperature is above its Tg. Lastly, like many polyolefins, PP is highly flammable and behaves essentially as a solid fuel, often requiring the use of flame retardants to enhance its safety in specific applications.
Polypropylene, while a versatile homopolymer, can be further tailored for specific applications through copolymerization, creating materials with enhanced or modified properties. These copolymers involve the incorporation of one or more comonomers alongside propylene units within the polymer chain. The chemistry, structure, and resulting properties of these copolymers are largely dictated by the type and amount of comonomer(s) used and the way these comonomers are incorporated into the polypropylene backbone.
One primary category is random copolymers, where the comonomer units, typically ethylene, are distributed randomly along the polypropylene chain. The introduction of these ethylene units disrupts the regular crystallinity of the homopolymer, leading to a lower degree of crystallinity and consequently, a lower melting point and increased flexibility and toughness, particularly at lower temperatures. The amount of ethylene incorporated usually ranges from 1 to 7%. These random copolymers retain good clarity and processability, making them suitable for applications like pipes for hot and cold water, transparent packaging, and fibers. The random distribution of the comonomer units prevents long sequences of propylene units from forming highly ordered crystalline regions.
Another significant class is block copolymers, also known as impact copolymers. These materials consist of polypropylene homopolymer segments linked with segments of a different polymer, often an ethylene-propylene rubber (EPR). The structure here is more of a sequential arrangement rather than a random distribution, Figure 5. The polypropylene blocks provide stiffness and high-temperature resistance, while the elastomeric EPR blocks dispersed within the matrix enhance impact strength, especially at lower temperatures. The size and composition of these blocks can be precisely controlled to achieve a specific balance of stiffness and impact resistance. These copolymers are widely used in automotive parts, appliances, and rigid packaging where toughness and impact performance are critical. The distinct phases of the hard polypropylene and the softer rubber contribute to their enhanced impact properties by dissipating energy upon impact.
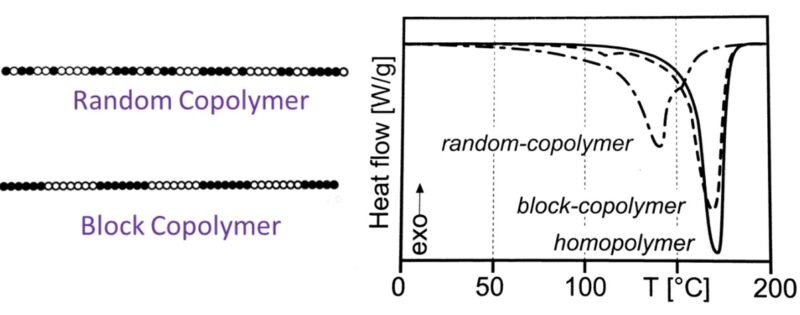
Figure 5. Structure of copolymers versus melting behavior.
Finally, there are terpolymers, which incorporate three different monomer units. These are less common than random or block copolymers but offer the possibility of even more finely tuned properties. For instance, a terpolymer might include propylene, ethylene, and a third monomer like butene to achieve a specific combination of flexibility, impact strength, and processability. The arrangement of these three monomers can be random or in blocks, further expanding the range of achievable properties. The specific choice of the third monomer and its incorporation method allows for highly specialized property profiles tailored for niche applications.
Cyclic Olefin Copolymer (COC/COP)
Cyclic Olefin Copolymers (COCs) are a class of amorphous thermoplastics synthesized by copolymerizing cyclic olefins, such as norbornene or tetracyclododecene, with linear olefins like ethylene. This distinctive chemical architecture (Figure 6), characterized by the incorporation of rigid cyclic units within the polymer backbone, bestows upon COCs a unique profile of properties that distinguishes them from conventional polyolefins like polyethylene (PE) and polypropylene (PP).

Figure 6. The chemical structures of COC and COP.
Notably, COCs exhibit high transparency, low density, minimal water absorption, exceptional dimensional stability, significant stiffness and strength, good chemical resistance (particularly to polar solvents, acids, and bases), and biocompatibility. Furthermore, they possess a high Tg that can be adjusted based on the specific copolymer composition. In essence, COC (sometimes referred to as COP, implying a polymer rather than just a copolymer) can be viewed as a unique type of polypropylene derivative featuring a closed-loop molecular arrangement. This amorphous structure results in a transparency comparable to polycarbonate (PC) and polymethyl methacrylate (PMMA), coupled with enhanced heat resistance, higher oxidation resistance, and sterilizability. However, these advanced properties come at the cost of increased material expense and a greater degree of brittleness when compared to standard polypropylene.
Key characteristics of COCs include the following:
- Amorphous: Transparent, similar to PC and PMMA.
- Increased Heat Resistance: Up to 160 °C.
- Higher Oxidation Resistance: Compared to PP.
- Sterilizable: Autoclave, EtO, gamma.
- Excellent Moisture Resistance: Suitable for demanding environments.
- PP Chemical Resistance: Retains much of PP’s chemical resistance.
- Pure Plastic: Low leachables/extractables, no BPA.
Despite its advantageous attributes, COC/COP also presents several limitations. Its cost is notably higher compared to standard polypropylene. Additionally, it exhibits a higher density, typically greater than 1 g/cm³. Mechanically, COC/COP tends to be brittle, with a relatively low strain at break of approximately 2%. While its gas barrier properties are an improvement over standard polypropylene, they are still considered low for certain demanding applications. Furthermore, processing COC/COP can be challenging, often requiring higher processing temperatures and a narrower processing window. Finally, its lower melt flow rate (MFR), or alternatively high viscosity, can influence and complicate various processing parameters.

Figure 7. The chemical structure of PMP.
Polymethylpentene (PMP)
Polymethylpentene (PMP), also known as poly(4-methyl-1-pentene), stands out as a unique polyolefin due to its regular isobutyl side groups along the polymer backbone, Figure 7. This structural feature distinguishes it from common polyolefins like PE and PP, and lacks the cyclic structure found in COC. This structural arrangement imparts a specific set of properties, most notably its exceptional transparency, often rivaling glass and surpassing other semicrystalline polymers, making it ideal for applications demanding high clarity. PMP also boasts the lowest density among all thermoplastics, enabling lightweight component design. Its chemical resistance is generally good to excellent, comparable to polypropylene in many respects, though it may be more vulnerable to certain unsaturated and aromatic hydrocarbons and chlorinated solvents. Characterized by a high melting point (around 230-240°C) and good form stability at elevated temperatures, PMP is suitable for applications involving higher heat exposure and even sterilization. Furthermore, it exhibits favorable dielectric properties, including a low dielectric constant and dissipation factor, making it useful in high-frequency electrical applications and offers excellent moisture resistance. However, despite these advantages, PMP is more brittle and possesses higher gas permeability compared to both PP and PE, which can be a limitation depending on the application.
Key characteristics of PMP include the following:
- Semicrystalline: Highly transparent (65% semicrystalline).
- High Transparency: The copolymer is extremely clear.
- High Chemical Resistance: ~<PP.
- Extremely Low Density: 0.833 g/cm3.
- High Tm and Tg: Tm 230 °C; Tg 30 °C.
- Sterilizable: Autoclave, EtO, gamma.
- Excellent Moisture Resistance: Suitable for demanding environments.
- Low Leachables/Extractables: High purity.
Despite its unique advantages, PMP also presents several drawbacks when compared to other polyolefins. It tends to be more brittle at ambient temperatures and exhibits lower impact resistance than many grades of PE and PP. Its cost is significantly higher than that of these commodity polyolefins. While PMP offers good chemical resistance, it may be more susceptible to strong oxidizing agents than PP, and its UV resistance is considered poor. Although it has a high melting point, its heat distortion temperature under load can be similar to that of polypropylene, necessitating careful consideration for structural applications at elevated temperatures. Additionally, PMP’s high gas permeability, while advantageous in some contexts, can be a significant disadvantage for packaging applications requiring strong barrier properties. Furthermore, compared to COCs, PMP exhibits lower oxidation resistance, and its thermal stability can be a concern in certain applications. Overall, its higher cost and increased brittleness, coupled with its higher gas permeability than PP and PE, limit its use to specialized applications where its unique combination of transparency, low density, and high melting point are essential.
Conclusions
Polyolefins are a highly versatile and widely used family of polymers with applications spanning packaging, automotive components, construction materials, medical devices, and consumer goods due to their broad range of properties (Figure 8), low cost, ease of processing, and chemical resistance.
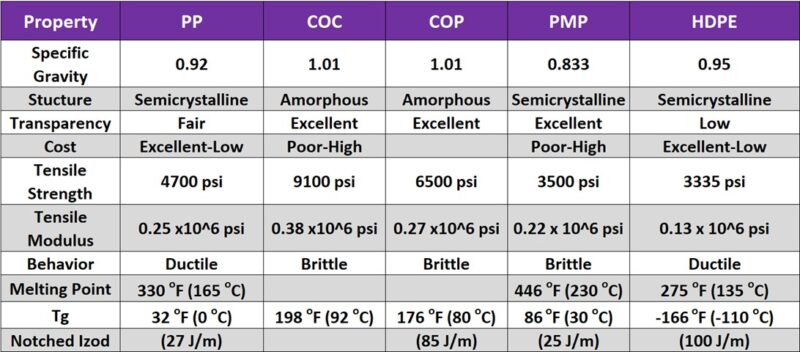
Figure 8. Typical properties of polyolefins.
However, their susceptibility to oxidation, creep under sustained loads, and inherent flammability necessitate careful consideration, often requiring the use of additives like antioxidants and flame retardants. Processing challenges can also arise with certain types like UHMWPE and COC/COP. When selecting a polyolefin, factors such as material cost (considering specific gravity), the potential use of reinforcements (which can impact knitline strength), and the time and temperature dependence of their properties are crucial for ensuring optimal performance in the intended application.
Despite their inherent limitations, polyolefins remain highly attractive materials across numerous industries, finding widespread use in diverse applications such as packaging (films, bottles, containers, and closures), automotive components (bumpers, dashboards, interior trim, and fuel tanks), construction (pipes, films, and insulation), medical devices (implants, surgical devices, and drug delivery systems), and a wide array of consumer goods (toys, furniture, and storage containers).
