This article studies the failure of a weighted medicine ball that cracked during use in an exercise drill that included repeated bouncing. Medicine balls are commonly used in training and for rehabilitation. Their weight, size and ability to bounce vary across the market and suit multiple needs. For instance, while the addition of weight is beneficial for strength training, bouncing balls allow for more athletically-based workouts. This evaluation identified features that were characteristic of failure occurring through low cycle fatigue associated with the bouncing of the product during use. Cracking of the medicine ball may have resulted due to a combination of low air pressure and uneven thickness distribution throughout the elastomeric materials in the shell of the product.
What Went Wrong?
The Madison Group (TMG) analyzes hundreds of product failures every year. In-situ experiments are often conducted to evaluate the contribution towards failure from material selection, manufacturing, design, intended use and installation practices. However, the failure described in this study, occurred outside of the TMG laboratory facility. Inadvertently, an uncontrolled fatigue experiment took place in the employee fitness room where a medicine ball was used for a slam exercise. In this exercise drill, a medicine ball is thrown down towards the ground. A slam exercise is meant to increase core and functional strength. The amount of force and frequency used for a slam exercise would vary depending on the user’s physicality. The medicine ball in this analysis was a weighted ball and would bounce when making contact with the ground when used for this exercise.
Failure was observed after a repetitive slam exercise. Failure affected the structural integrity of the medicine ball and reduced its ability to bounce. The failure event of the ball did not result in any injuries or property damage. However, it prompted questions about the construction and failure of this piece of fitness equipment.
References about the product indicated that the medicine ball’s construction allowed it to bounce off of hard surfaces. The ball included an air valve. This indicated that the air pressure inside of the ball aided towards the balls’ bouncebackability following impact. It should be noted that the air pressure and frequency of use for the failed ball were not monitored throughout its lifetime. It is unknown whether the ball would bounce or not when deflated.
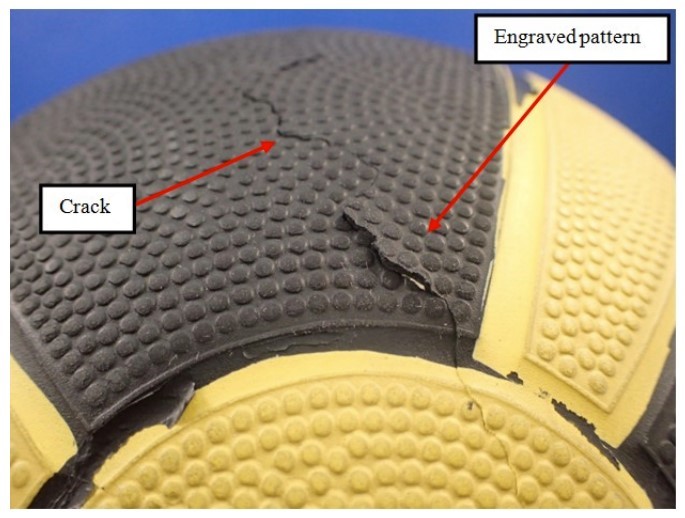
Figure 1-View of the continuous crack on the exterior of the medicine ball.
Failure Evaluation
A single, continuous crack was observed on the exterior of the medicine ball. The crack appeared to extend through almost half of the circumference of the ball and followed a continuous, but irregular pattern with no visible bifurcations (Figure 1). The external surface of the ball featured an engraved pattern. However, the crack propagation did not appear to be affected by the external, engraved features nor by any other feature on the exterior, such as color or location relative to the ball’s air valve. To further study the crack development, destructive analysis was conducted. This analysis included sectioning of the ball, microscopy and material testing.
No evidence was found to indicate that damage, such as abrasion, gouging or cutting contributed to the failure of the medicine ball. The ball’s ability to bounce was notably reduced after the failure and attempts to pump the ball up, showed it was deflated. In order to evaluate the medicine ball’s construction and to further study its failure mode, the medicine ball was cross-sectioned.
Sectioning of the medicine ball was performed at a location near the observed crack to allow for further examination and exposure of the fracture surface. The sectioning of the ball revealed an internal bladder and a shell composed of three layers of material; (First Layer, Second Layer and External Layer) (Figure 2).
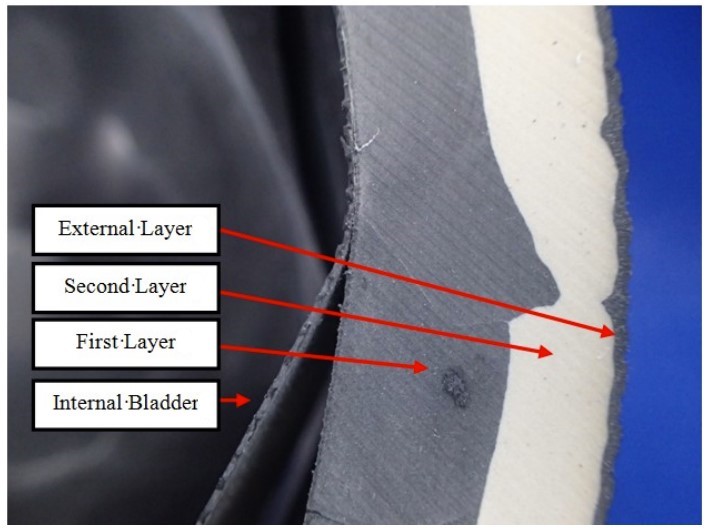
Figure 2 – View of the internal construction of the medicine ball.
No sudden or loud noise was reported at the time of the cracking. This could indicate that the air pressure inside of the ball was low or that the ball was already deflated at the time of failure. The bladder showed a rupture in the region adjacent to the main crack location (Figure 3).
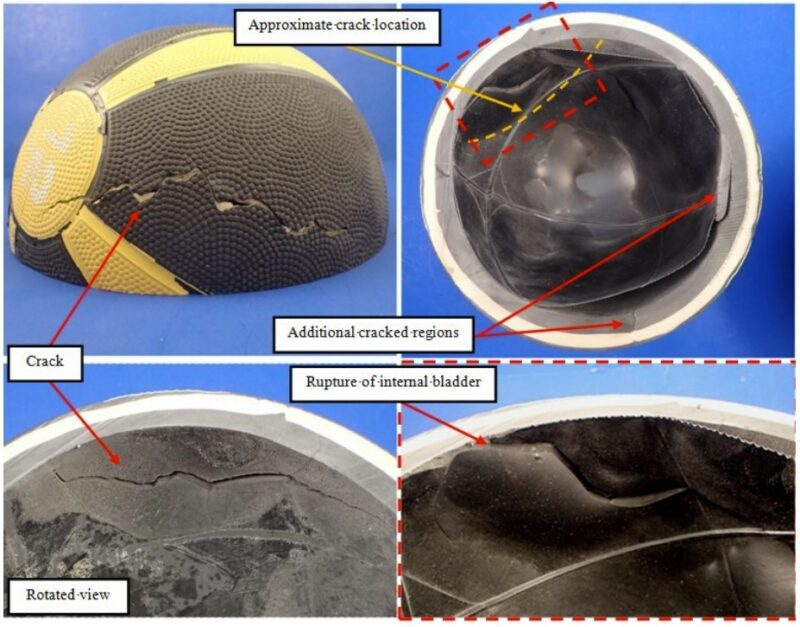
Figure 3 – Views of the cross-sectioned medicine ball showing the crack location, the ruptured internal bladder and additional cracked regions observed on the First Layer of material.
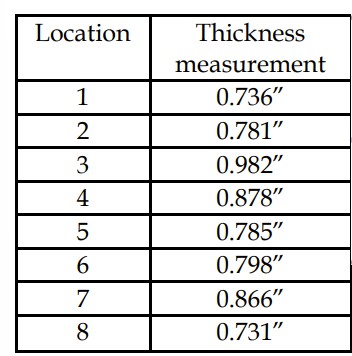
Table 1 – Thickness measurements on the cross-sectioned medicine ball.
Therefore, it is possible that the rupture of the internal bladder was a post-failure event due to the effects on the structural integrity of the ball from the shell’s failure.
Thickness measurements on the cross-section showed that cracking occurred at a region with reduced thickness along the circumference of the medicine ball (Table 1). The nature and causes of the thickness variation were associated with the manufacturing defects of the medicine ball. The presence of thickness variations can reduce the overall strength of the product, and could have made this region prone to crack development and failure.
The region of failure showed no visual signs of material defect, degradation or aging. Microscopic examination of the crack surface showed a single crack origin location on the interior of the First Layer of material. The crack initially propagated radially through the shell. Subsequently, the crack extended circumferentially in both directions (Figure 4). This pattern of crack progression was also observed through the Second Layer and External Layer of material. The observed fracture features were consistent with a progressive failure mechanism, as indicated by the presence of parallel indications. The form and relatively lower number of observed indications were thought to represent crack propagation through low cycle fatigue.
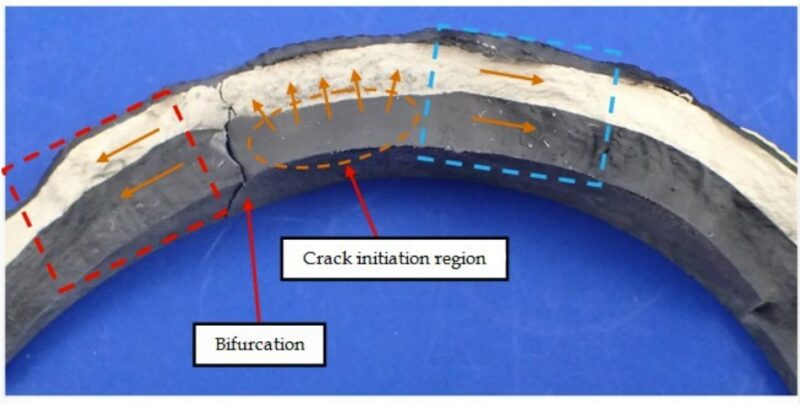
Figure 4 – Exposed fracture surface of the medicine ball with arrows indicating the direction of crack propagation.
Additional cracking on the First Layer was observed as a bifurcation of the main crack. Furthermore, two additional crack locations were identified on the First Layer at remote locations from the main crack (Figure 3). These cracks had only progressed through the First Layer of material. The progressive cracking may be an indication that after the cracks had propagated sufficiently to relieve stress, continued cracking occurred through low cycle fatigue associated with subsequent bouncing of the medicine ball.
Through Scanning Electron Microscopy (SEM), it was possible to observe the progressive cracking features and significant amounts of filler material in the First, Second and External Layers (Figure 5).
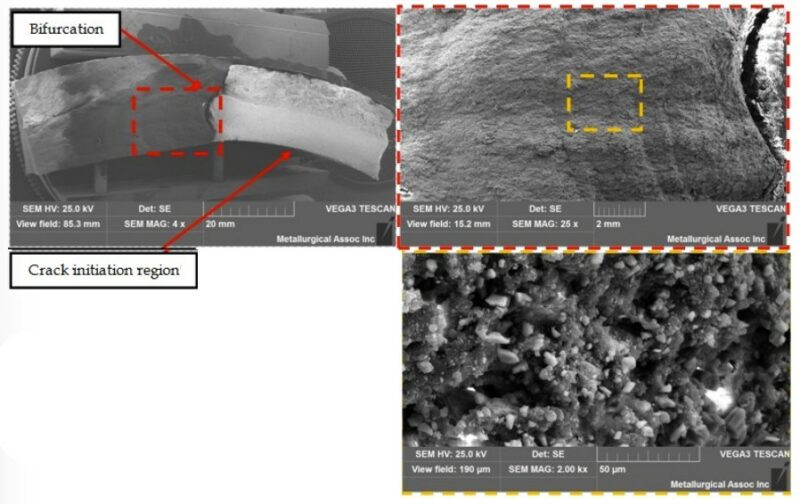
Figure 5 – Scanning electron micrograph views of the exposed fracture surface.
The high magnification views did not reveal additional features on the crack progression through the materials in the medicine ball. Analysis of the materials in the medicine ball via Differential Scanning Calorimetry (DSC) produced very similar results. The thermograms indicated that the materials underwent endothermic events that covered a broad temperature range, centered at approximately 90 °C during the first heating run. The totality of the DSC results obtained for the medicine ball components were indicative of thermoset materials. The thermal events were thought to be attributed to the continued curing of the thermoset resins (Figure 6).
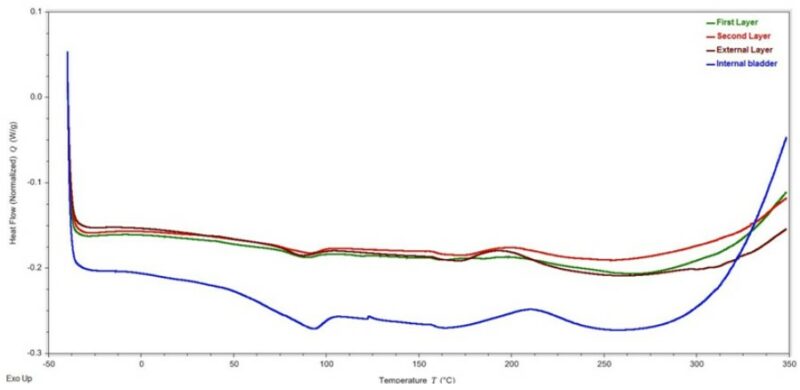
Figure 6 – Initial heating DSC thermogram of the components of the medicine ball.
The Fourier Transform Infrared Spectroscopy (FTIR) spectra obtained for the components of the medicine ball indicated that the internal bladder and the First, Second and External Layers of material consisted of styrene-butadiene based materials. Furthermore, the spectra showed absorption bands characteristic of calcium carbonate (CaCO3), a common filler in rubber formulations (Figure 7).
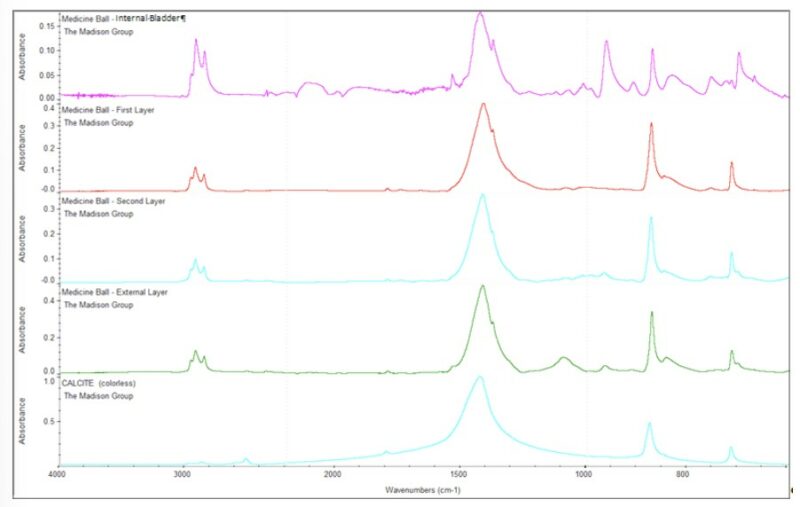
Figure 7 – FTIR spectra of the components of the medicine ball. Each of the components exhibited absorption bands characteristic of styrene-butadiene based materials. Furthermore, the spectra showed absorption bands characteristic of calcium carbonate.
On comparing the thermogravimetric analysis (TGA) results for the various layers in the medicine ball, it can be seen that both the internal bladder and the First Layer decomposed in a similar manner. Similarly, the Second and External Layers of the material showed comparable TGA profiles. The initial weight loss observed across the TGA profiles for all of the materials, was associated with the volatilization of hydrocarbon-based plasticizers within the material’s formulation. The test also showed that the material for the First, Second and External Layers were identified as having approximately 70% of filler content, while the material in the internal bladder showed filler content of approximately 26% (Figure 8). Based on the results from the FTIR testing, a larger portion of the filler content could be attributed to a calcium carbonate filler.
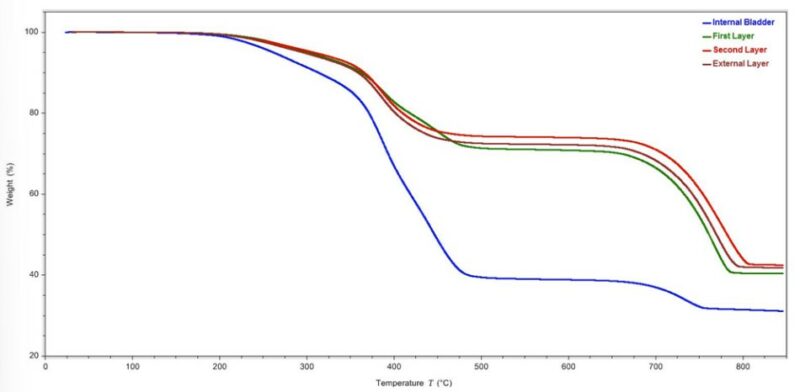
Figure 8 – TGA thermogram of the components of the medicine ball.
Based on the material analyses performed on the components of the medicine ball, it is likely the internal bladder was made out of an SBR rubber based material while the layers in the shell of the ball were likely, EPDM rubber based materials.
Conclusions
This analysis showed that the failure of the medicine ball occurred through a low cycle fatigue mechanism. This resulted in the development of a large crack that propagated through the First, Second and External Layers of elastomeric materials in the ball. Sectioning of the ball revealed evident variations in thickness throughout the shell of the medicine ball with cracking initiating at a region with reduced thickness. Given the unknown air pressure at the timeframe when failure occurred or the amount of stress and frequency for the applied stress, it is likely that a combination of these factors caused the failure of this piece of fitness equipment during use.
