Polymers are macromolecules that are based on a structure built-up, chiefly or completely, from a larger number of similar structural units bonded together. Often called chains, the polymer consists of repeating units, similar to links. Polymers are formed through a process known as polymerization, in which monomer molecules are bonded together through a chemical reaction that results in a three-dimensional network of long individual polymer chains consisting of smaller repeated units.
There are two basic types of polymerization reactions: addition and condensation. Addition polymerization is the formation of polymers from monomers containing a carbon-carbon double bond through an exothermic addition reaction. Significantly, this reaction proceeds without the loss of any atoms or molecules from the reacting monomers. Common materials produced through addition polymerization include polyethylene, polypropylene, poly(vinyl chloride), and polystyrene as represented here:
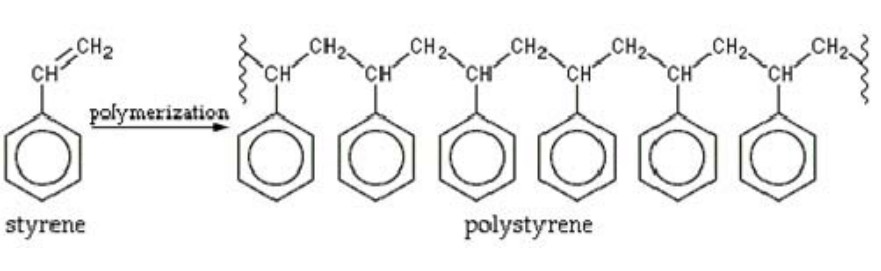 In contrast, condensation polymers are formed by a stepwise reaction of molecules with different functional groups. The reaction is endothermic and produces water or other small molecules such as methanol, as a byproduct. Common polymers produced through condensation reactions include thermoplastic polyesters, polyacetal, polycarbonate, and polyamides as represented here:
In contrast, condensation polymers are formed by a stepwise reaction of molecules with different functional groups. The reaction is endothermic and produces water or other small molecules such as methanol, as a byproduct. Common polymers produced through condensation reactions include thermoplastic polyesters, polyacetal, polycarbonate, and polyamides as represented here:

Addition polymers form high molecular weight chains rapidly, and tend to be higher in molecular weight than condensation polymers. Comparing polymers produced via the two different mechanisms, addition polymers are generally, chemically inert due to the relatively strong carbon-carbon bonds that are formed. Condensation polymers tend to be susceptible to hydrolytic molecular degradation through exposure to water at elevated temperatures, through a mechanism that resembles the reversion of the initial liberalization reaction.
Polymers produced through these two different types of polymerization mechanisms exhibit inherently different characteristics, including mechanical, thermal, and chemical resistance properties. As such, it is important to understand the type of polymer being considered in an application.
