This paper was originally published at the Society of Plastics Engineers Annual Technical Conference 2013.
Abstract
Many chemicals have the ability to attack plastics leading to failure. In some cases, the source of the chemical is not well defined. In this study, the effect of biodiesel, a fatty acid methyl ester, on various plastics, namely polyamide 6 (PA 6), polycarbonate (PC), acrylonitrile-butadienestyrene (ABS) and ABS/PC plastic blends was studied. Various feedstocks of biodiesel were also studied, including, soy bean oil (new and used), animal fat (tallow), corn oil as well as choice white grease. The plastics samples were tested following an ASTM standard where a predefined strain is applied to the samples prior to exposure to the solvent (biodiesel). This study has shown that biodiesel can be incompatible with engineering plastics, such as PC, ABS and ABS/PC blends.
Introduction
In the recent decade, there has been a continued rise of biofuel production in the United States due to the fluctuations of petroleum costs and negative impacts of fossil fuels to the environment. This has demanded the creation of a more sustainable energy resource such as bioethanol and biodiesel. The biodiesel industry, in particular, has produced more than 1 billion gallons in 2011 [1]. The U.S. National Biodiesel Board has noted that it is a key milestone for the industry. As of August 2012, there are about 699 million gallons of biodiesel produced according to the U.S. Energy Information Administration [2]. Currently, there are 105 biodiesel plants in the U.S. with an annual production capacity of 2.1 billion gallons. It is expected that these numbers will continue to increase as new feedstocks resources are explored.
The use of vegetable oils in diesel engines was considered inadequate due to its high kinematic viscosity, which can lead to engine fuel deposits. Vegetable oils have to be reduced to a lower viscosity before it can be used as a diesel fuel. One of the methods to obtain this is by transesterification. In the presence of a catalyst (acid, base or enzyme), triglycerides in oils reacts with an alcohol (typically methanol is used), to produce biodiesel or fatty acid methyl esters (FAME) and glycerol. This involves three reversible stepwise chemical reactions that convert triglycerides to diglycerides to monoglycerides, then finally to glycerol. Each step releases one mole of FAME. The transesterification process decreases the viscosity significantly, correspondingly giving the biodiesel a viscosity that is low enough to be used in diesel engines.
Standard #2 diesel fuel is a liquid diesel fuel derived from petroleum. It is mainly composed of saturated hydrocarbons and aromatic hydrocarbons. To be used as substitute for #2 diesel fuel, biodiesel needs to meet ASTM D6751 and EN14214 before it can be used as a substitute for standard #2 diesel. These standards give detailed requirements (e.g. flash point, water and sediment content, other contaminant levels) that the fuel must meet.
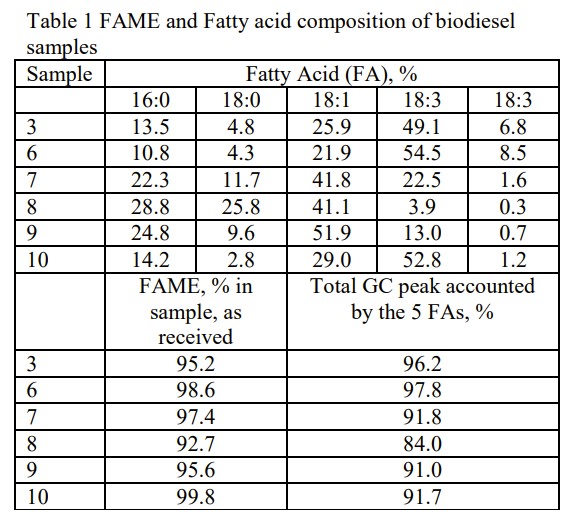
Biodiesel fuel is made from a reaction of triacylglycerides and methanol. The resulting biodiesel is essentially fatty acid methyl esters (FAME), as shown in Figure 1. It is known that many plastics will have incompatibility issues with ester-based chemicals [3]. The study investigated the compatibility of various plastics to six biodiesels. formulated from different feedstocks.
Chemical Degradation
Chemical degradation of plastic by a foreign material involves the reduction in length of the molecular chain and corresponding molecular weight of the polymer. It is generally observed that the longer the molecular chains of the plastic, the more desirable the mechanical properties, as well as other properties [4]. This is primarily due to the entanglement of the long molecular chains. Chemical degradation involves a chemical reaction where the molecule is modified and effectively reduced in length.
Contributors include: David Grewell, Tong Wang, Melissa Montalbo-Lomboy, Linxing Yao, Iowa State University, Ames, IA; Paul Gramann and Javier Cruz, The Madison Group, Madison, WI
