This chapter originally appeared in ASM Handbook, Volume 11B, Characterization and Failure Analysis of Plastics, published by ASM in 2003
Many fracture mechanisms exist that can lead to failure of a plastic component; however, environmental stress cracking (ESC) is recognized as one of the leading causes of plastic failure. It is estimated that approximately 25% of plastic failures are attributed to ESC (Ref 1). The ESC mechanism of failure is prevalent throughout most industries and market sectors. Some examples of plastic parts commonly seen in day-to-day failure analysis investigations that fail from ESC include:
- Housings and enclosures for medical devices
- Assemblies joined with metal screws
- Poly(acrylonitrile-butadiene-styrene), poly(vinyl chloride), and chlorinated poly(vinyl chloride) pipes
- Automotive lenses
- Handheld devices
- Architectural and multilayer glazing
Lately, ESC failures have become increasingly prevalent in the medical device industry due to the changes in cleaning methods and aggressiveness of new disinfection solutions. While certain ESC failures could be attributed to changes in an industry that were unforeseen by designers and manufacturers, ESC has been a long-standing issue that has resulted in numerous failures in plastic components over the years. Part of the reason for this is a lack of awareness and understanding of the mechanism and of the complexities involved in being able to properly characterize it.
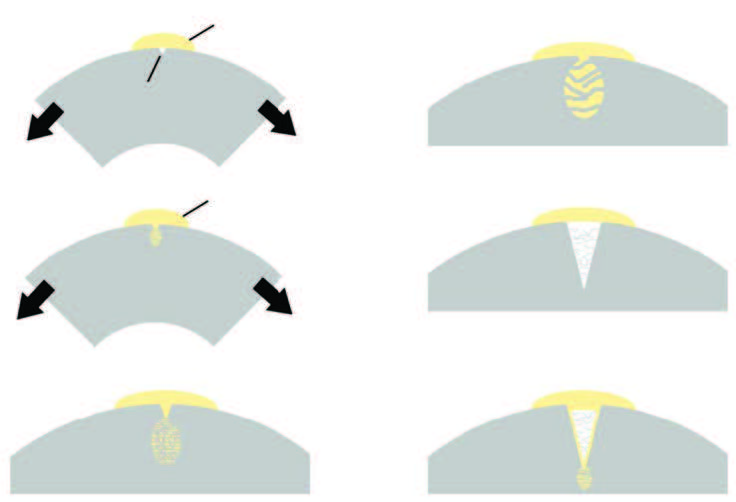
Generalized schematic of the steps involved in the formation of an environmental stress-cracking (ESC) fracture.
Environmental stress cracking is a mechanism of fracture that results from the simultaneous and synergistic exposure of a plastic to chemical and stress. To properly understand the mechanism, it is important to have a basic knowledge of the molecular structure of plastics and how they can crack. As shown in the simplified schematics in Fig. 1, the polymer within the plastic recipe is made up of multiple long-chain molecules. Their long lengths allow the molecules to interact. Depending on the shape and orientation of the molecules and the attraction forces between them, they may form crystalline and/or amorphous regions. Crystalline regions result from greater intermolecular forces that lead to tightly knit and organized structures called lamellae (Fig. 2) (Ref 3). These lamellar structures are comprised of polymer molecules that have created organized folding patterns upon cooling. As the polymeric molecules move, fold, and interact, some regions entangle but are not able to crystallize. Ultimately, the material organizes into a unique conformation of amorphous and semicrystalline regions that is dictated by the material chemistry and processing conditions. The polymer backbone chemistry will have the greatest effect in the ability of a material to form crystals or remain amorphous.
The atoms that form the backbone of the polymer are all attached together by covalent bonds, resulting in very strong backbones that are difficult to break. Chain scission, known as the action of breaking these covalent bonds, requires that the molecules be exposed to high energy levels. These may result from high shear heating, very aggressive chemicals, UV energy, or other sources of high energy. The mechanism of ESC varies from other chemical fracture mechanisms, such as chemical degradation, in this specific aspect: the covalent polymer backbone bonds do not need to be affected for ESC to take place. The craze formation that initiates an ESC mechanism of fracture results from the slippage and disentanglement of polymeric molecules rather than by chain scission. In essence, the ESC mechanism parallels creep. Creep in plastics occurs due to the natural slippage and disentanglement of the molecular structure when a part is under load. The same process holds for ESC. Although for the case of ESC, the environment includes a chemical exposure that accelerates the disentanglement mechanism.
Individual long-chain molecules in a thermoplastic are held together by means of molecular entanglement and by lower-energy intermolecular forces of attraction, such as van der Waals forces, London dispersion forces, hydrogen bonding, and dipole interactions. Figure 3 provides an example of such forces for a polyamide 6,6, showing how polymer molecules that are next to each other will be attracted to each other as a result of hydrogen bonding. During an ESC failure, the chemical interacts with the less-organized amorphous regions of the polymer, causing a reduction of these intermolecular forces and leading to molecular slippage and craze formation.
Environmental stress cracking (ESC) is a mechanism whereby failure can occur while the material is exposed to lower levels of energy and less-aggressive chemical interactions. No bulk solvation or plasticization is necessary for ESC to occur. For a chemical to have an effect on the polymer, the chemical must have a certain affinity to the polymer so that it can penetrate between the molecules, occupy free volume, and interfere with the polymer-to-polymer attractions, thereby reducing these attractions. In general, these polymer-to-polymer intermolecular forces are strong enough and the free volume is small enough that a chemical cannot easily migrate in, unless there is a significant attraction to the polymer, such as what would be expected from a solvent or plasticizer. However, because ESC chemical agents are less aggressive (have less attraction to the polymer), they rely on a compounding factor to ease their entry into the polymer. This factor is stress.
When a plastic material is stressed, the orientation of the molecules changes. Intermolecular forces of attraction counteract against the stress. Stressed locations that are under tension become regions where the molecules are effectively being pulled apart. The resulting stress effect allows the chemical to interact and alter the polymer structure more easily. For semicrystalline thermoplastics, a possible scenario for introducing this mobility was described by evaluating intrinsic crazing, which is crazing induced solely by stress and not accelerated by chemical effects (Ref 4). It is suggested that when a stress is applied, free volume can increase in local regions where the polymer is under stress. In fact, molecular orientation, such as that created by polymer flow, plays a significant role in this phenomenon. If stress is applied parallel to the orientation direction of the polymer molecules, ESC resistance can be increased by factors of up to four times, as reported for poly(methyl methacrylate) (Ref 5). Conversely, for stresses applied perpendicular to the direction of orientation, the opposite effect can occur. An easy way to understand this is to imagine that polymer molecules that have been oriented during processing are like a bundle of strings oriented in a similar axis. Pulling on the string bundle perpendicular to their length will simply open the space between them, effectively increasing their free volume.
This chapter was authored by Javier C. Cruz and Jeffrey A. Jansen.
