This article originally appeared in Plastics Engineering Magazine – December 2015
ESC, “the plastic killer,” is a frequent cause of field failures.
Environmental stress cracking (ESC) is considered a leading cause of plastic part failure. It is the premature embrittlement and subsequent cracking of a plastic due to the simultaneous and synergistic action of stress and contact with a chemical agent. Because of the frequency and severity of ESC failure, it has been nicknamed “the plastic killer”.
A principal consideration in the relatively high failure rate associated with environmental stress cracking is the widespread lack of awareness and understanding of the interaction between plastic materials and chemicals, particularly organic-based chemicals, which are so prevalent in manufacturing, commercial, and household settings. Most failures associated with ESC occur through contact with a secondary chemical agent. A secondary chemical agent is one that is not anticipated to contact the molded plastic part throughout its lifecycle.
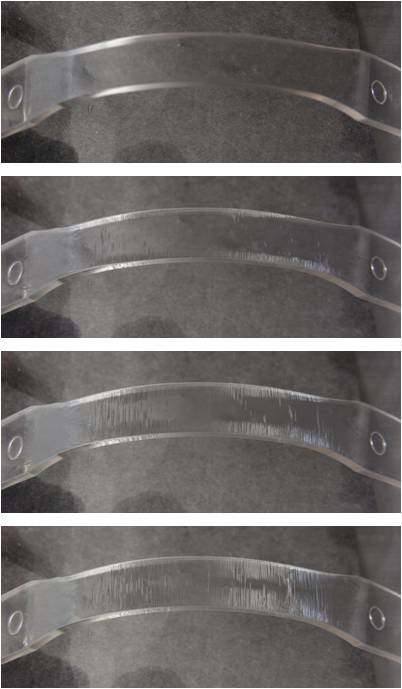
Time-lapse views showing ESC with polycarbonate.
This is in contrast to a primary chemical agent, where contact is expected, such as gasoline with a small-engine fuel filter, or paint with the tubing in a paint sprayer. Failures connected with primary chemical agents are rare in service because the chemical compatibility is well understood at the design phase. Failures associated with secondary chemical agents are far more common.
A common misconception about environmental stress cracking is that it involves molecular degradation or chemical attack of the plastic material. This is not true. The ESC failure mechanism does not proceed through a chemical reaction between the polymer and the chemical agent. Molecular structure alteration or degradation does not occur. Significantly, in the absence of the chemical the plastic would given sufficient time. The chemical simply accelerates the stress cracking. Because of this, environmental stress cracking and creep rupture are parallel failure mechanisms. Several factors will accelerate the embrittlement process associated with creep rupture, including an increase in temperature, stress concentration within the part, cyclic loading, fatigue, and contact with specific chemical agents (ESC).
In environmental stress cracking, the chemical agent permeates into the molecular structure of the plastic, interfering with the intermolecular forces bonding the polymer chains, allowing accelerated molecular disentanglement. This reduces the energy required for disentanglement to occur. The presence of a moderate environmental stress crack agent can result in an order of magnitude reduction in the time to failure.
An important consideration is that the chemical agent does not permeate substantially into the plastic material, and only the material on the contact surface is affected. The bulk properties of the material, including stiffness and strength, are not affected. Other chemically induced failure mechanisms, such as plasticization and solvation, include further interaction between the chemical agent and the plastic.
