Differential scanning calorimetry (DSC) is a thermoanalytical technique that measures temperatures and heat flows associated with thermal transitions in a material. The results are used to understand physical and chemical changes involving endothermic or exothermic reactions or changes in heat capacity. In thermoplastic and thermoset materials, these transitions could include the glass transition temperature (Tg), melting temperature (Tm), cold crystallization, crystallization during cooling, enthalpic relaxation, curing, and more.
Using this transition data, information can be obtained regarding the following:
- What annealing temperatures to utilize
- What the approximate melt temperature should be during processing
- How cooling will affect the material (mold coolant)
- If there are any adverse effects from processing (e.g., crystallization and degradation)
- What degree of crystallinity was obtained in a molded component
- Information regarding crystallization behavior
- What curing temperatures and times should be utilized
It is also important to understand these thermal transitions because the material will undergo significant changes in properties as it gets closer to and moves through the transition temperatures. This is due to the mobility of the molecules, which is commonly referred to as viscoelasticity. A viscous material, such as honey, will displace when a stress is applied. This viscous material will not recover its strain, and return to its original position when the stress is relieved. The material will become permanently deformed. In contrast, elastic materials, such as steel that undergoes displacement from a stress application, will return to its original position when the stress is relieved. Polymers exhibit both the elastic response (steel) and viscous response (honey). The amount of viscous and elastic responses are highly dependent on the application temperature in relation to the thermal transitions. The closer to the thermal transitions that a material is used, the more mobility the molecules will have to undergo a viscous response. In other words, they will respond more like honey and less like steel the closer they are to the thermal transitions.
During a typical DSC experiment of a polymeric material, the material starts to be heated below the thermal transitions being studied. The exact starting temperature is dependent on the material undergoing analysis, as well as the DSC’s ability to develop a baseline and equipment restrictions. As polymers undergo thermal transitions over a range of temperatures, starting and ending points are chosen to ensure the thermal transition will not be affected by the temperatures. Furthermore, the material is maintained below temperatures that will degrade or adversely affect the polymeric material.
As previously noted, the DSC is a powerful tool that can provide a lot of information by analyzing thermal transitions exhibited by the material. When analyzing materials, they will often exhibit multiple transitions (exothermic and/or endothermic) during testing. In some cases, these transitions can occur within the same temperature range, making interpretation of the thermal transitions and information more difficult. Some examples of this are enthalpic relaxation due to aging and molecular re-orientation, glass transition or crystallization and melting, which typically occur at the same temperature range. One solution to separate these overlapping transitions is to apply both a sinusoidal and linear heating/cooling rate to the sample through the use of Modulated DSC (MDSC).
Figures 1 and 2 show an example of the sinusoidal heating rate for an MDSC experiment. In this experiment, a sinusoidal heating rate that fluctuated from 6.0 °C/min to 0 °C/min, was applied to the sample. This was done so that an average heating rate of 3.0 °C/min would also be maintained.
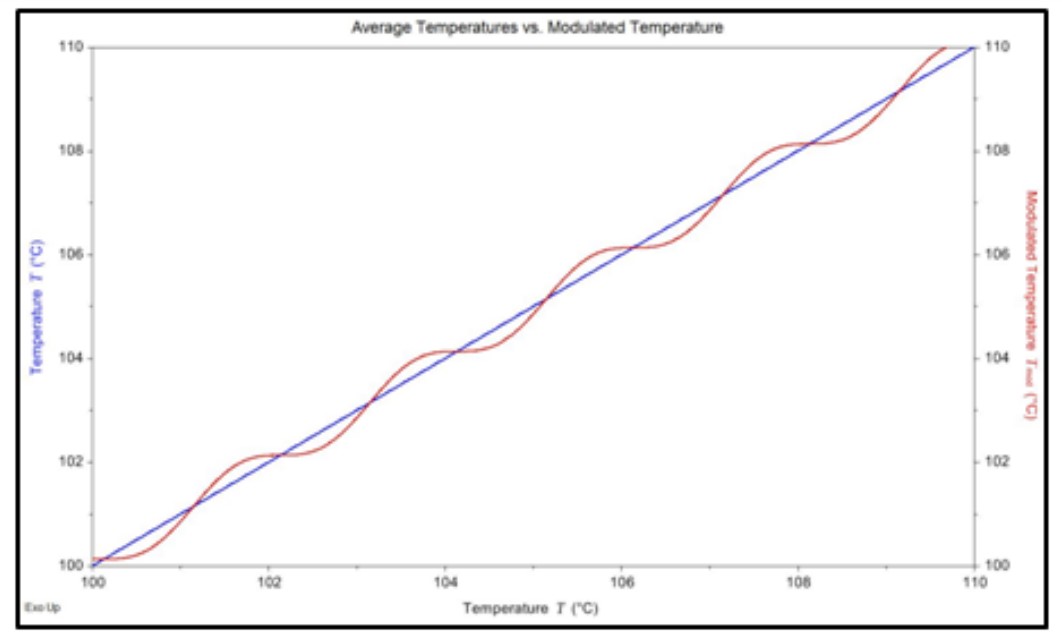
Figure 1. Comparison view the sinusoidal and average temperatures for MDSC.
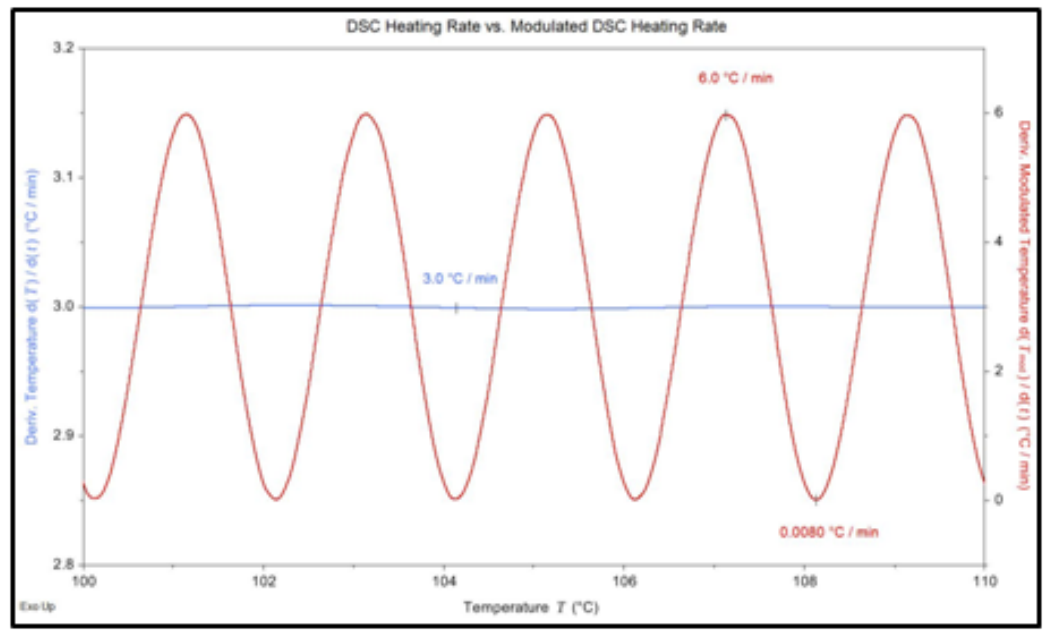
Figure 2. Comparison view of the sinusoidal and average heating rates for MDSC.
With both the average and modulated heat flows being observed, essentially two sample experiments were being conducted at once. The sample’s behavior could then be used to separate the irreversible transitions (cold crystallization and enthalpic relaxation) from the reversible transitions (Tg and Tm). This would allow for more accurate quantitative measurements from the data. Figure 3 shows the difference between a standard DSC and a MDSC thermogram.
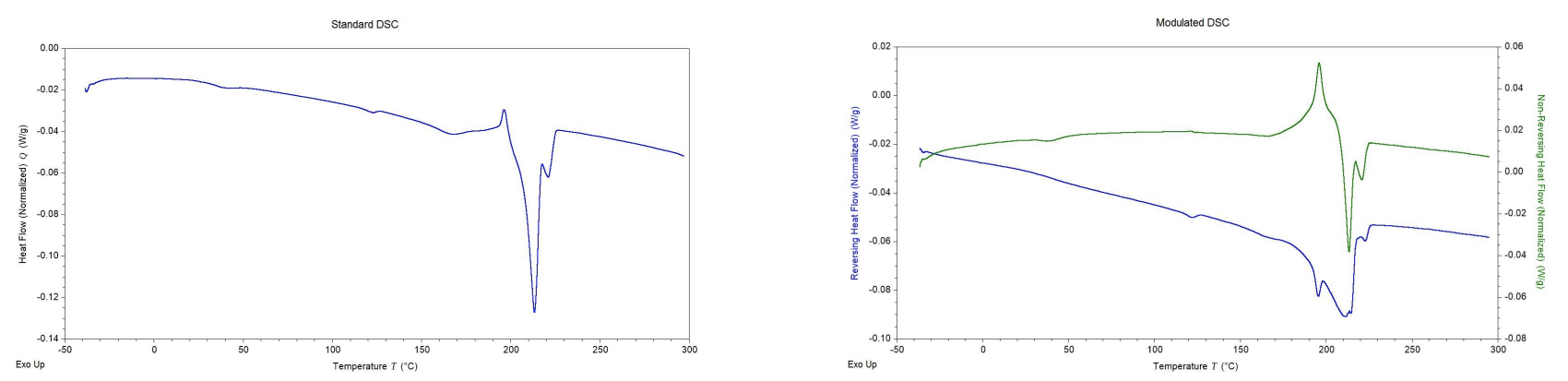
Figure 3. Comparison of a standard DSC thermogram versus a MDSC thermogram.
