There is nothing more frustrating to a polymer expert than when the failure of a plastic part happens to them firsthand. Adding to this frustration is knowing that the failure could have most likely been avoided. I can say this from experience.
It was a sunny morning, I was driving into the office when it happened. I went to adjust my cellphone in the holder mounted to the HVAC vent. As I was gently performing the adjustments the cellphone suddenly dropped onto the floorboard. When I arrived at the office, I took a closer look and observed that the holder had in fact separated into multiple pieces, Figure 1.
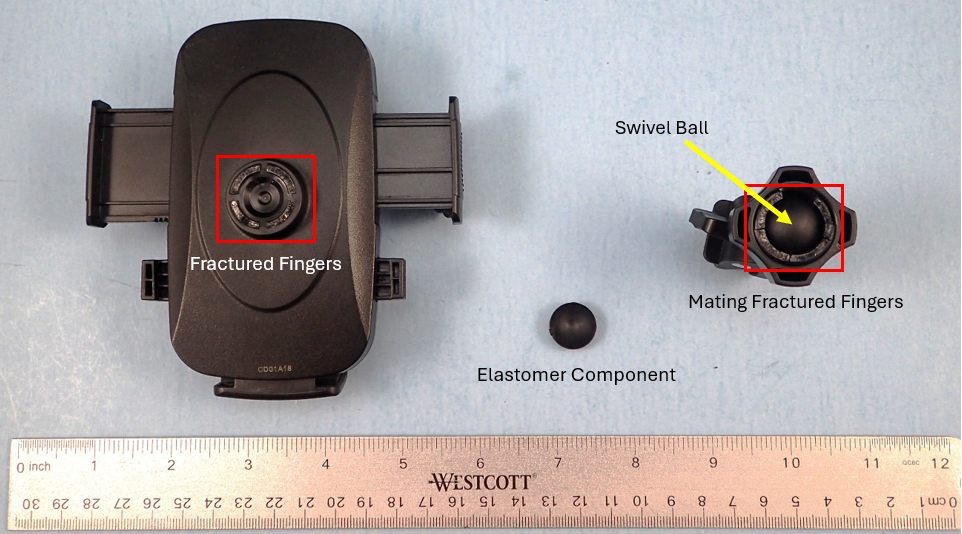
Figure 1. The cellphone holder components following failure.
The holder exhibited fracture at the location of four fingers that were to be securely holding onto the swivel ball. Additionally, because of the failure a small hemi-spherical elastomer component between the swivel ball and holder body had been freed. My curiosity grew and I needed to know what had caused this failure.
Compositional Analysis – Cellphone Holder
Multiple analytical techniques were used to determine the composition of the holder material, Figure 2. Fourier transform infrared spectroscopy (FTIR), used to analyze the organic bonds of the material, helped to determine that it was comprised of a polycarbonate resin. Differential scanning calorimetry (DSC), used to investigate the thermal transitions of the material, confirmed a glass transition temperature consistent with that of polycarbonate with no evidence of contamination. Thermogravimetric analysis (TGA) revealed that the polycarbonate resin was essentially unfilled.
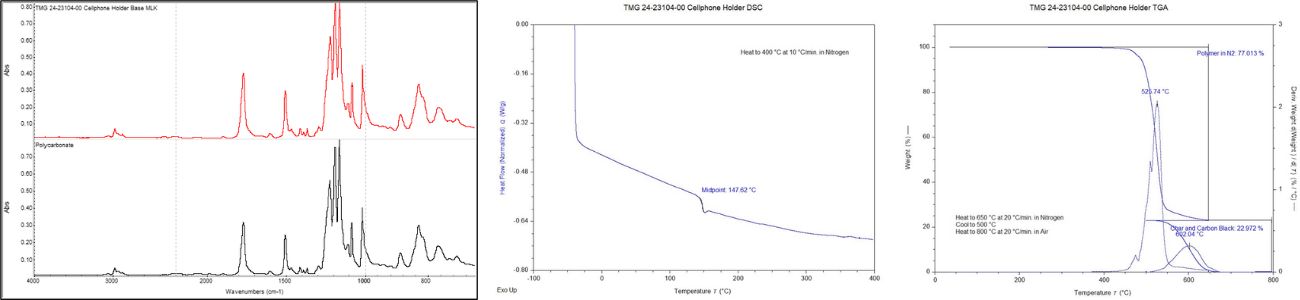
Figure 2. The results of FTIR, DSC, and TGA analyses on the cellphone holder.
Melt flow rate (MFR) testing was also performed to understand the quality of the molded polycarbonate material. This technique measures the flow rate under a specific temperature and load condition and can be used to determine if molecular degradation has occurred during processing. Typically to perform such a determination virgin resin of the polycarbonate material used to mold this part is required, or at a minimum a nominal MFR value for the resin provided. Unfortunately, neither of these were available, but it is known that most commercially available unfilled polycarbonate resins have melt flow rates that fall between 10 and 25 g/10 min. Based on this, it may be possible to make some assessment of the quality of the molded holder.
The results of the MFR test are provided in Table 1 and determined an average MFR of 13.265 g/10 min. This result indicated that the cellphone holder material was of good quality and, most likely, did not experience significant molecular degradation during the molding process.
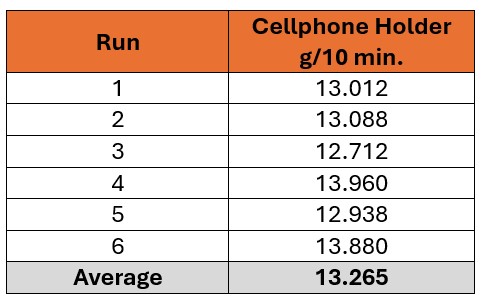
Table 1. Results of the MFR testing obtained at 300 °C and 1.2kg following drying to 71 ppm moisture content.
Fractography – Cellphone Holder
Since the material analysis of the cellphone holder itself yielded no clues as to why it had failed it was time to perform an examination of the fracture surface itself. The fracture surface was to be evaluated using scanning electron microscopy (SEM). This technique can be used to determine the origin of a crack as well as the mode of initiation and propagation. It will provide insight into the type of loading as well as possible contributions from chemical exposure or other environmental effects.
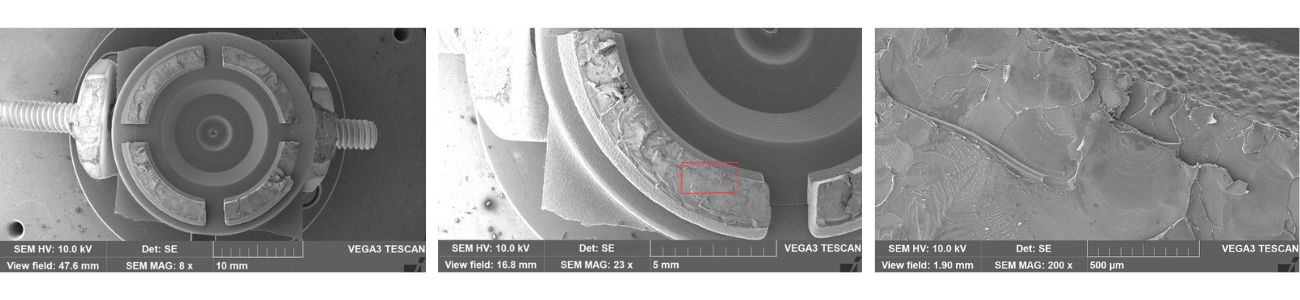
Figure 3. Microscopic images of the cellphone holder fracture surfaces.
The examination of the fracture surface is illustrated in the electron micrographs of Figure 3. In the overview of the fracture surfaces, the bottom left fracture surface was selected for higher magnification examination with the area of interest indicated by the red rectangle. This examination revealed multiple origins adjacent to the inner diameter of the finger. Adjacent to the origins were striations, consistent with a cyclic mode of crack propagation, as well as open craze remnants.
These features were characteristic of environmental stress cracking. Environmental stress cracking (ESC) is a failure mechanism whereby a plastic material cracks due to contact with an incompatible chemical agent while under stress. It is a solvent-induced failure mode, in which the synergistic effects of the chemical agent and mechanical stresses result in cracking. This result was curious as the cellphone holder had not been exposed to the treatment of any chemicals from myself in the application. Then I remembered the hemi-spherical elastomer.
Compositional Analysis – Elastomer Component
FTIR analysis was performed on the elastomer component to determine its composition. This test was conducted directly on the elastomer as well as its solvent extract residue. The analysis results revealed that the elastomer was comprised of a polyurethane-based resin with an ester-based plasticizer, such as acetyl tributyl citrate, Figure 4.
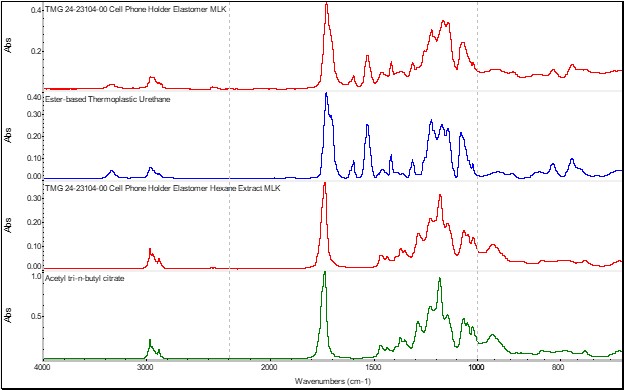
Figure 4. The FTIR results obtained on the elastomer component.
Polycarbonate materials have a well-documented history of experiencing ESC in the presence of ester-based plasticizers. So, the mystery was solved! The cellphone holder failed due to ESC cracking as the result of direct contact with another component, which utilized an incompatible chemical for a plasticizer. However, what is really at the root cause of this failure is that the product development team failed to consider the entire system. Specifically, they did not understand that a component within the assembly contained an ingredient that was chemically incompatible with another component within the same assembly. Every day, there are numerous failures that can be avoided simply by expanding chemical compatibility assessments beyond the application chemicals to include components within the assembly itself.
Learn more about environmental stress cracking here.
