Polyurethane materials may be one of the most versatile polymers in existence. This three-part series highlights this incredible material through an understanding of its structure and diverse properties.
If you were to ask ten engineers to name something manufactured from a polyurethane material, you may very well receive ten different responses. To one, it may be the hard, protective coating they use as a wood finish. To another, it may denote the rigid foam insulation for weatherproofing or the soft foam underlayment for carpeting. Polyurethane materials can be used to manufacture running shoes, upholstery, cushioning, suspension bushings in heavy equipment, in-line skate wheels, flexible tubes/film/sheets, soft-touch overmolded features, adhesives, binders, coatings as well as automotive spoilers, bumpers and internal features.
Polyurethanes can be prepared as cross-linked thermosets that are cured through two component systems, thermoplastic elastomers that can be melt processed (and re-processed) or even foamed structures that can be soft and flexible or rigid materials. Though the properties can vary dramatically, some general benefits of polyurethanes can include:
• Excellent resistance to oils, greases and many solvents
• High elasticity/flexibility over a wide range of temperatures
• Abrasion and wear resistant
• Tear resistant and good compression set
• Good weather and UV resistance
• Ease of coloring
• Good tactile properties
• Wide range of processing methods
• Good bonding/adhesive properties
• Excellent impact and rebound properties
Given the tremendous range of applications and diverse properties of polyurethanes, it is often convenient to categorize them according to their hardness. Though it is tempting to correlate some mechanical properties with hardness, to do so would be misleading and incomplete. (A more thorough review of properties and behavior will be discussed in Part 2 of this series) The tremendous range of hardness available in commercial polyurethanes span from soft rubbers to hard thermoplastics. How can a single synthetic material find so many different applications and take so many forms? The simple answer to this diverse range of properties and behavior can be traced back to its roots – chemistry.
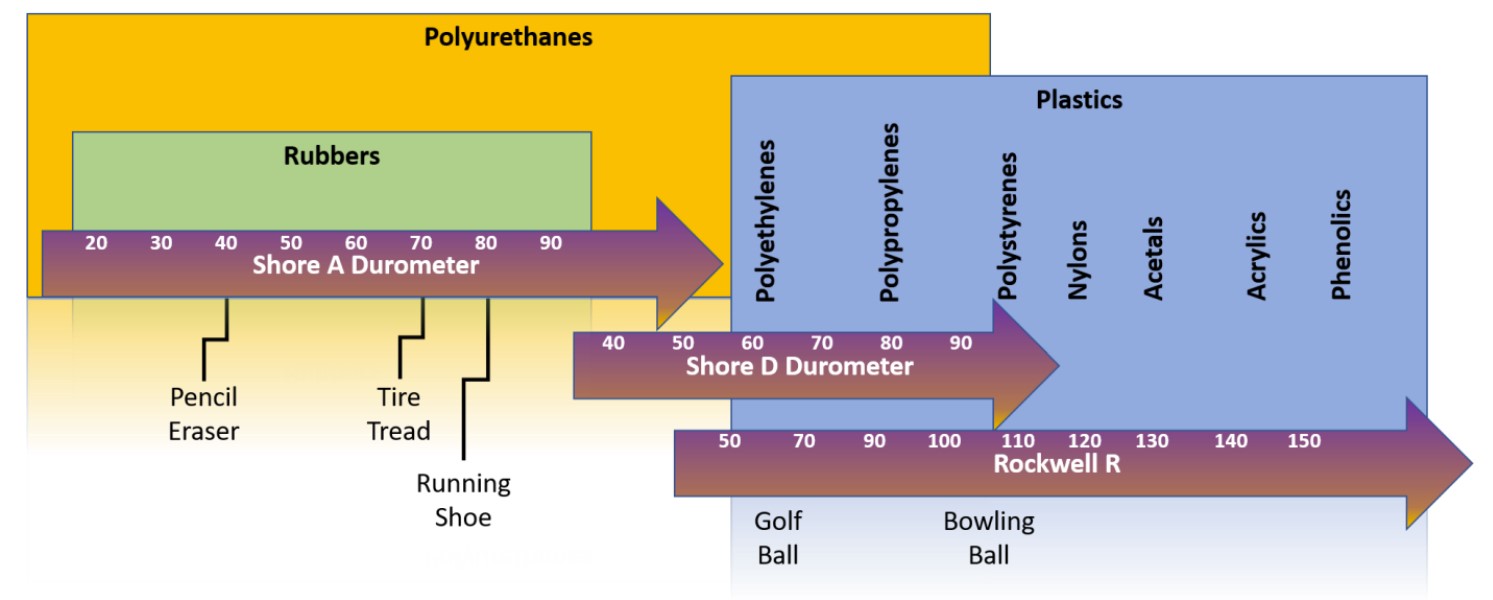
Hardness scales for various materials and products.
Often considered a “necessary evil” to a polymer engineer, chemistry forms the basis for all polymer structures – and ultimately defines many of their properties. Yet, one does not need an intimate understanding of the stoichiometric calculations of reactants, additives and byproducts necessary to synthesize a polyurethane. Instead, a basic understanding of the underlying chemistry will suffice to appreciate the broad spectrum of behaviors found in polyurethanes.
Polyurethane was originally patented under the work of Otto Bayer and his coworkers at IG Farben in Leverkusen, Germany in 1937 while they reportedly were trying to copy a Nylon 6,6 structure. The single-claim patent discloses the method to produce polyurethane by reacting a polyol (-OH group) with a diisocyanate (-NCO group). The specific choice of these base chemistries (along with a suitable chain extender and additives), their relative ratios and the way they are assembled are what ultimately will control the final properties and behavior of the polyurethane material.
A polyurethane molecule can be considered as a block copolymer that is constructed from three basic constituents: a polyol, a chain extender and a diisocyanate. The specific chemistry of these constituents as well as the way in which the resulting soft and hard block segments are arranged provide toughness as well as flexibility for the elastomer. The hard segments within the polyurethane molecules can order with one another to form crystalline regions. For thermoset polyurethanes the same ordering may also occur but additional functionality within the curative will produce cross-linked chemical reactions between the individual molecules. For both thermoplastic and thermoset varieties, it is this carefully planned ordering that offers polyurethanes the tremendous range of mechanical properties and behavior.
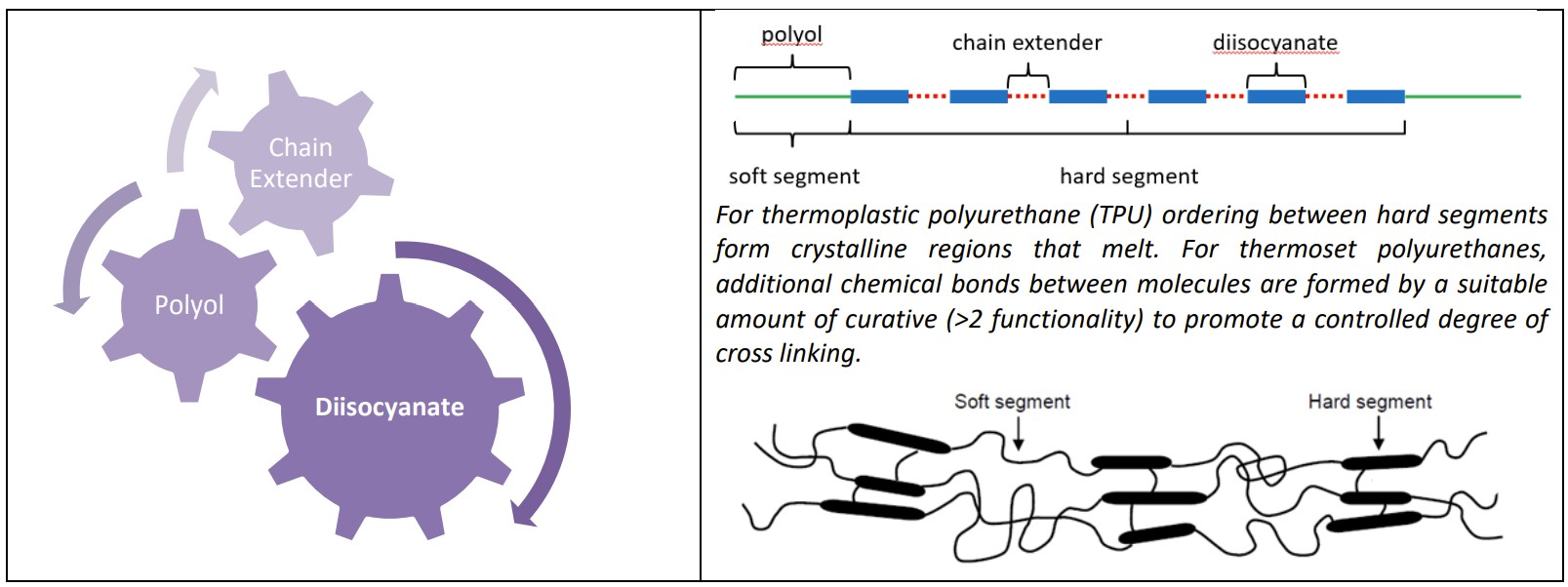
Polyurethane molecule construction
Additionally, many of the mechanical and physical properties exhibited in a polyurethane material are directly affected by the specific chemical choices for the polyol, diisocyanate and chain extenders. Though beyond the scope of this introductory white paper, each of these constituents can be broadly categorized in terms of their chemical classes, bonds and ring structures. Though other hybrids and options are available, the polyols used for most commercial polyurethanes can be described as either polyether- or polyester-based. Similarly, the diisocyanates used during the synthesis of polyurethane materials can be generally classified by their ring structures as aliphatic or aromatic. Finally, the chain extenders, low molecular weight (short chain) diols or amines align themselves to connect with the stiff hard segments. For thermoset polyurethanes, higher functionality (>2) polyols and amines act as crosslinkers between the molecules.
Therefore, it is the combination of the base chemistry of the “ingredient” components used for the polyurethane, as well as the order/manner in which they are assembled onto the molecule that control the material’s properties. Ultimately, it is the complex interaction between the soft and hard segments in a polyurethane system that provides the desirable physical properties such as elasticity, tensile strength, tear resistance, and elongation. However, the details of how these properties are developed and used is left to the next installment of this white paper series on Polyurethanes: Where Rubber Meets the Road.
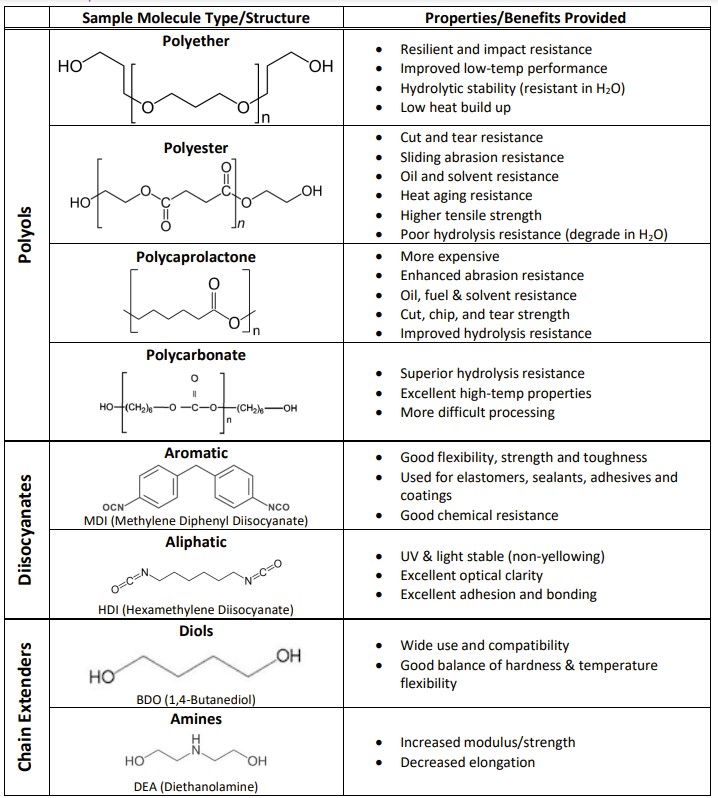
Table showing some typical polyurethane constituent chemistries and their effects on properties.
