For the material analyst, some of the most powerful techniques to evaluate polymeric materials are thermal analysis techniques. Examples of these tests are thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), thermomechanical analysis (TMA), and dynamic mechanical analysis (DMA). The theory behind these testing techniques is to evaluate how the material changes as the temperature is varied. Specifically, the dependent variables in these experiments are weight change, heat flow, dimensional change, and mechanical property change. With this data, one can start to obtain a picture of the material behavior and structure.
When evaluating a problem that arises during design, manufacturing, or end use of a product, there are a seemingly endless number of testing methods used to evaluate and characterize materials. However, in the real world, limitations in sample size, budget, or timeline can affect available testing options for most projects. Unfortunately, these restrictions do not adjust the goal of the project, which is to solve the issue. In these cases, the power of thermal analysis becomes evident with the small sample sizes and wealth of data received. This data, when properly analyzed, can be used to investigate the “what” and the “why” of many problems typically encountered in the industry today.
As with anything, the success of analyzing the results of thermal analysis relies on a strong application and understanding of the fundamentals of polymer science/ chemistry. The Webster’s dictionary definition of fundamental is an original source; serving as a basis supporting existence; or determining essential function. A more concise set of synonyms for a fundamental would be primary, central, origin, and absolute. In athletics, the fundamentals would be how you move your body in order to throw a ball, hit a pitch, or score a basket. However, in polymer science, molecular structure is king. The molecular structure dictates the properties of a material. Thus, it is with a strong understanding of molecular structure, the fundamental, that a deep understanding of thermal analysis results is achieved.
Figure 1 – The molecular structure is the fundamental building block that dictates how the material behaves when heated and the mechanical properties of the material.
Before moving forward let us consider the antonyms of fundamental, which are defined as secondary, consequential, or dependent. Relating to sports, examples of this could be the impact position of the golf club or baseball bat. This impact position is not fundamental in that it will vary based on how you move your body leading up to that moment in time. Thus, in order to fix your “banana slice,” you must focus on the fundamental motion of your body rather than the secondary outcomes. In polymers, the majority of the reported properties of materials are a consequence of the micro-structure of the material. In thermal analysis, the temperature at which numerous different transitions/ material changes occur has been tested and catalogued for many known polymers. Examples of these properties would be glass transition, melting temperature, melting enthalpy, decomposition temperature/onset, along with many others. However, when you consider all of these properties you see that these are not absolute, but instead are a consequence of the fundamental structure of the material, Figure 1.
Let us consider a single property, glass transition (Tg), to understand how this phenomenon applies to the chemical structure. The glass transition temperature is extremely important to both the short-term and the long-term material properties of a polymer. In short, the glass transition is the temperature at which localized molecular freedom of motion is attained. Below the glass transition, the polymer molecular motion is limited and thus, the material behaves in a brittle, glassy manner. However, above the glass transition the material has freedom of motion at a local level, which makes the material behave in a rubbery manner. A classic example of these property differences is a racquet ball. At room temperature, the ball will bounce due to the rubbery state of the polymer molecules. However, if the ball is submerged in liquid nitrogen (brought below glass transition) and thrown at the ground, it will shatter. Factors that will affect the glass transition, from a purely polymeric standpoint, would be the types of bonds (stiffer backbone increases Tg), types of side groups (larger molecules increase Tg), molecular interaction (polarity and length of chains), and other polymer factors like branching or cross-linking. Having this basic knowledge can help significantly in understanding the thermal transitions observed in the thermal analysis. Further, this information will help to piece together why a material shows variations in the thermal transitions. Take for example the difference in glass transition of approximately 20 ° C between polycarbonate and poly(phthalate carbonate). The difference in backbone structure includes an additional ring structure that increases the backbone stiffness, Figure 2. This difference, in part, results in an increase in the glass transition temperature of the material.
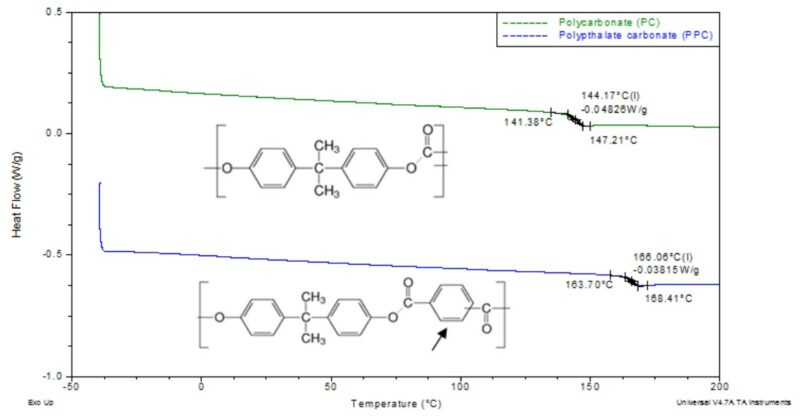
Figure 2 – Comparison of DSC thermograms for two polycarbonate-based materials showing how the backbone structure can affect the glass transition.
Understanding the fundamentals of what affects the physical and thermal properties of a polymer requires “dipping your toes” in the world of polymer chemistry. This can be a daunting and terrifying task, at first. However, having knowledge of the basics is an extremely powerful problem-solving tool. Like having solid fundamentals in sports, it gives you something to fall back on in order to fix the issues that are being observed on the back end. This bottom-up approach is powerful in solving the most difficult problems we face as we can get to the heart of the matter and work our way up from there.
To truly understand the “why” in a problem-solving situation, it is important to convert the “secondary” symptoms of the issue to the fundamental. Unfortunately, the problems that arise in the industry are almost always manifested as symptoms. We see failures that occur because the material does not have the correct stiffness, the strength was too low, it cracked over time, etc. It is the job of the problem solver to take a step back and convert the symptoms into the fundamental issues at hand. This technique ensures that you are not just treating the symptoms of the problem, but rather, attacking the underlying cause.
