The molecular structure of polymeric materials leads to temperature dependency and critical thermal transitions.
This temperature dependency within plastics is directly attributable to the viscoelastic nature of polymeric materials. As the temperature is increased, the polymer chains are further apart, there is more free volume and kinetic energy, and the molecules are more mobile and can slide past one another and disentangle more easily.
Because of this molecular mobility, polymers will pass through key transitions, including the glass transition temperature (Tg), over a unique temperature range. The physical properties and performance of polymeric materials, such as strength, stiffness, and impact resistance, are highly dependent on the temperature at which the stresses applied., particularly above and below the glass transition temperature. Understanding the implications of these transitions and their correlation to molecular structure is useful in material selection and avoiding premature failure.
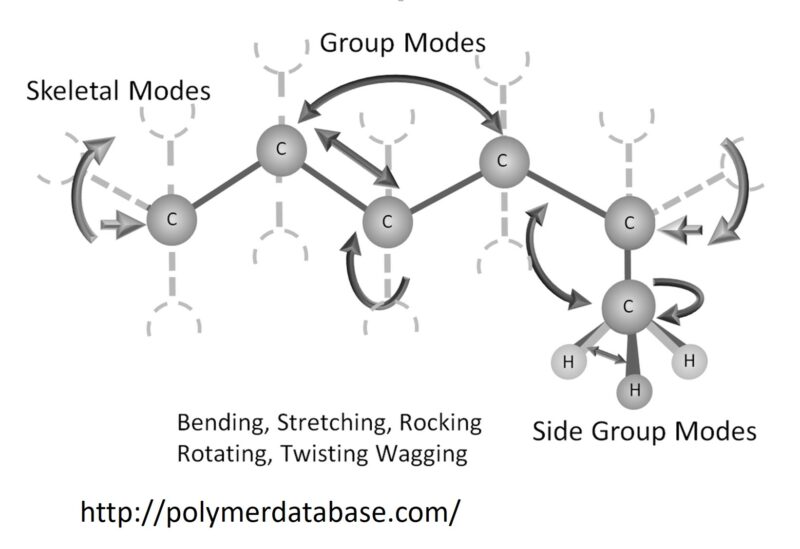 The glass transition temperature (Tg) represents the temperature at which the forces holding the molecules comprising the amorphous segments of a polymer together are overcome, so that the individual polymer chains are able to undergo large-scale molecular motions.
The glass transition temperature (Tg) represents the temperature at which the forces holding the molecules comprising the amorphous segments of a polymer together are overcome, so that the individual polymer chains are able to undergo large-scale molecular motions.
Transitional temperature of polymer chain stiffness or mobility from glassy state to flexible state. In other words, glass transition temperature (Tg) is the temperature when the molecules within a polymer chain begin to be in motion.
The kinetic theory of glass transitions states that as the material is heated, the glass transition (Tg) is reached. At this temperature, large scale coordinated motions of the polymer chains occur and a dramatic change in properties is observed.
The glass transition temperature (Tg) of a polymer is dependent on the molecular structure. For this reason, polymers have different glass transition temperatures.
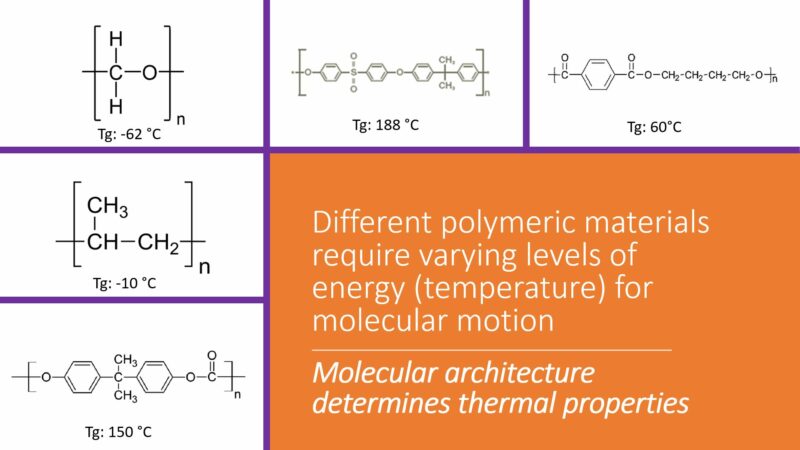
There are a number of molecular structural factors that play a role in the glass transition temperature (Tg). Three stand out as the primary molecular characteristics that will affect this thermal transition.
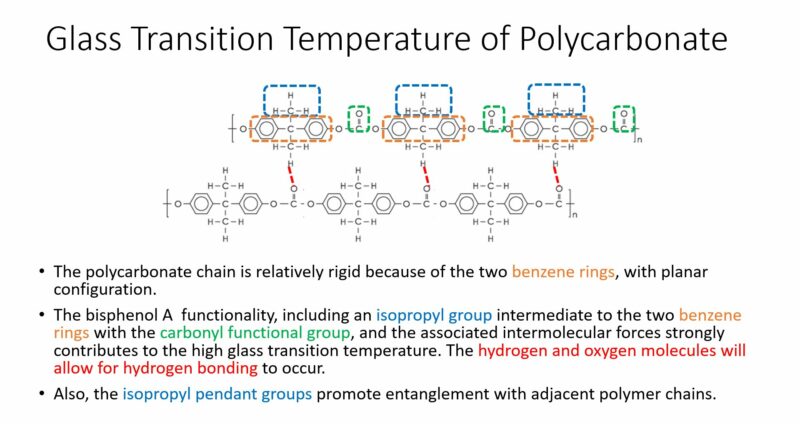
Polymer chain mobility. Generally, the more immobile the chain, the higher the glass transition temperature. Thermodynamically, a polymer chain that can move easily, relative to other entangled chains, will change from a glass state to a rubbery state at a relatively low temperature.
Inherent stiffness of the polymer chain. Functional groups that serve to stiffen the chain will reduce the flexibility of the polymer molecule, in turn raising the value of the glass transition temperature.
Level of intermolecular forces. The greater the level of intermolecular forces that hold the polymer chains together, the higher the glass transition temperature will be for analogous polymers.
The molecular structure characteristics with determine the glass transition temperature or polycarbonate are presented in the graphic below.
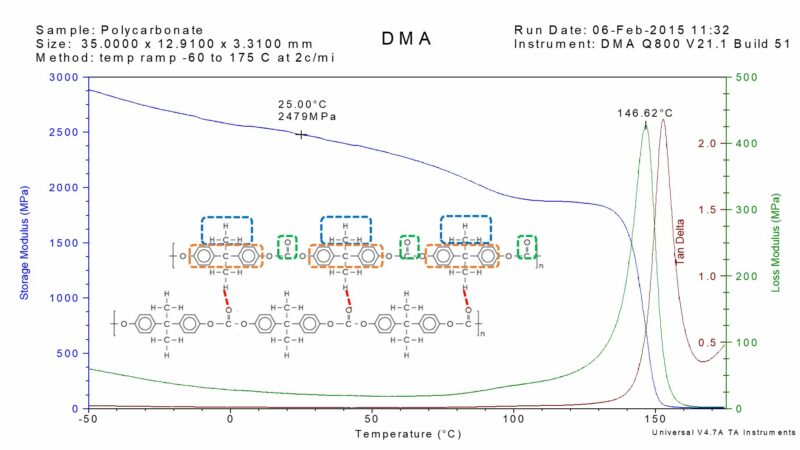
Dynamic mechanical analysis (DMA) provides the best measurement technique for the glass transition temperature (Tg). It is a direct assessment of molecular changes within the material. The glass transition temperature is typically assigned as the peak temperature in the loss modulus curve. This corresponds well with other thermal analysis techniques. The assignment of the glass transition temperature for polycarbonate is illustrated in the DMA temperature sweep thermogram illustrated below.
To learn more about how the glass transition temperature is assigned using DMA, check out this White Paper: Determining Glass Transition Temperature Using DMA
