The power of polymers is in their molecular weight. It is critical that manufacturers not only mold defect-free parts that meet print dimensions, but also ensure proper molecular weight retention of the polymer during processing. For hygroscopic polymers, this includes ensuring that the resin has been appropriately dried below the maximum recommended moisture content. Without appropriate drying, the performance and appearance of the molded parts will be comprised and parts that should work successfully in the end application could experience avoidable failure.
This article will begin by exploring the mechanics of water absorption in plastics. It will continue by reviewing how to properly dry resins, including appropriate techniques to determine their moisture content. Finally, it will conclude by reflecting on how by not properly preparing resins, can adversely affect the performance of the molded part.
Water Absorption
Most polymers during the synthesis, transport, and storage tend to retain moisture. However, this water absorption is reversible. The amount of moisture retained is based on an equilibrium value with the environment. This equilibrium value depends on the type of polymer, humidity and air temperature, pellet geometry as well as other factors.
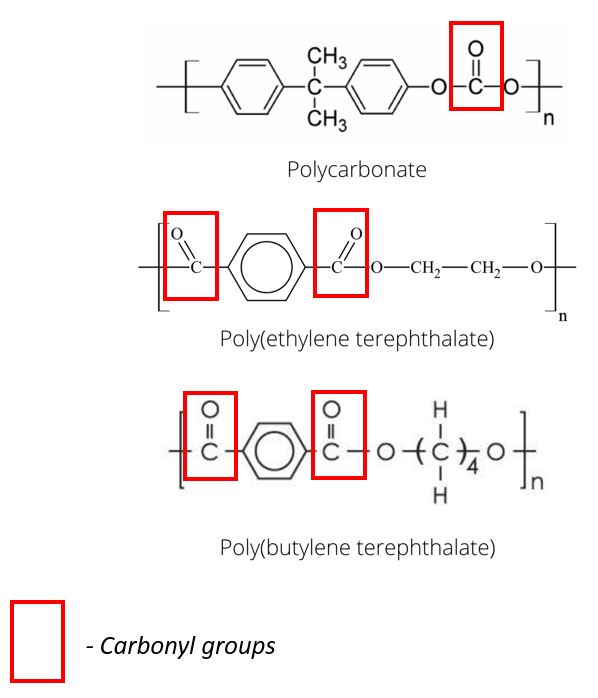
Figure 1. Examples of carbonyl groups for a variety of polymer structures.
When it comes to water absorption behavior, polymers can be classified into two types: hygroscopic and non-hygroscopic. For hygroscopic polymers, water is absorbed inside the plastic pellet and is chemically bound to the material itself. This type of polymer includes, but is not limited to: polyamides (aka nylons), polycarbonate, poly(methyl methacrylate), poly(ethylene terephthalate), poly(butylene terephthalate) and poly(acrylonitrile -butadiene-styrene). Among hygroscopic polymers, there are differences in the amount of water that can be absorbed. Since the water chemically binds itself to the polymer, these differences are driven by the chemistry variations in the polymers themselves.
Polymers containing many carbonyl groups, such as polycarbonate, poly(ethylene terephthalate), and poly (butylene terephthalate), will have a partial negative charge on the oxygen atom, which attracts the partial positive charge on the hydrogen atom of the water molecule. For those of you who are not chemists, a carbonyl group is essentially, a carbon atom double-bonded to an oxygen atom, Figure 1.
Polyamides not only have carbonyl groups that will attract water, but they also contain a nitrogen-bound hydrogen. This nitrogen-bound hydrogen has a partial positive charge, as the nitrogen atom is much more electronegative than the hydrogen atom. This positive charge acts to attract the negative oxygen charge of the water molecule, Figure 2.
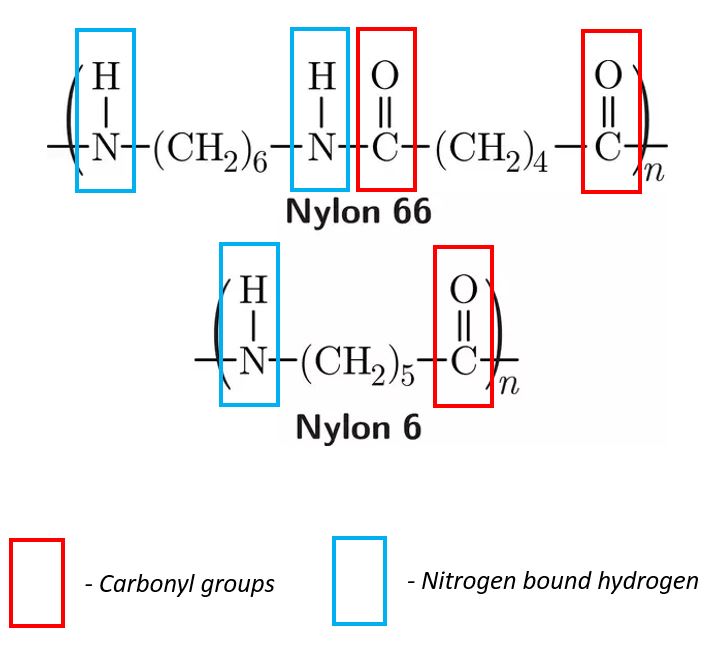
Figure 2. Examples of carbonyl groups and nitrogen bound hydrogens in nylons.
For non-hygroscopic polymers, water does not penetrate the pellets but rather settles on their surface. This type of polymer includes, but is not limited to: polyethylene, polypropylene, and polystyrene. Given that this type of water retention is purely a surface effect, the associated retention levels are typically much lower than hygroscopic polymers.
It is important to keep in mind that the incorporation of compounding ingredients, including certain additives, mineral fillers and fiber reinforcement can also affect a plastic material’s affinity for water absorption and retention. The water retention of a plastic pellet is also heavily dependent on its surface area for both hygroscopic and non-hygroscopic polymers. This is one of the primary reasons why regrind materials absorb more moisture, faster than virgin resin.
This retained moisture content in plastic pellets can result in several issues during processing, including:
• Viscosity shifts that impact process stability.
• Cosmetic defects in the molded part, such as bubbles, flash, splay or silver streaking.
• Inferior part performance due to hydrolysis of the polymer.
Drying
Some resins are dried prior to packaging while others are packaged with a moisture level greater than the maximum level recommended for processing. Most virgin resins are shipped in bags or boxes that are designed to reduce the rate of moisture absorption, but do not stop moisture absorption altogether. Once resin is received and its bag or box is opened, its moisture level can change rapidly. Any drying performed prior to leaving the manufacturer can be quickly undone. This makes it critical that resins are prepared in accordance within the resin manufacturers guidelines. Most resin manufacturers provide guidance for drying of their materials on corresponding datasheets or processing guides. Typically, that guidance comes in the form of a recommended drying temperature, drying time, and maximum moisture content, Table 1. While the recommended drying temperature may be an absolute, the drying time is merely directional and assumes that the resin is at some nominal moisture level when drying is initiated.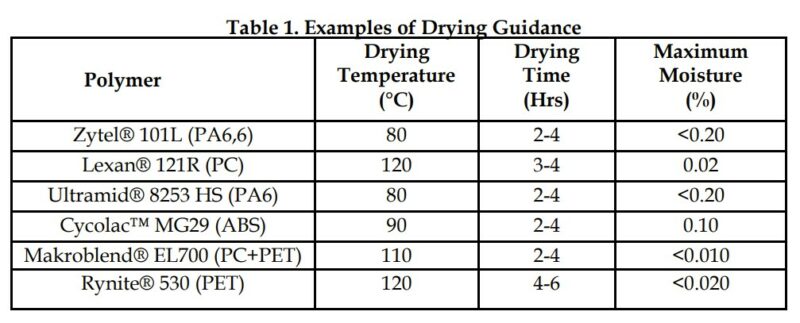
While guidance on the drying duration may be a good place for the molder to start, it is best to let the moisture level of the resin determine how long the resin should be dried for. This can be achieved by monitoring the moisture level of the resin over the course of drying to ensure an appropriate level has been reached prior to molding. To remove surface moisture from non-hygroscopic resins, hot air dryers provide the simplest option. Hot air dryers operate on the same principle as a hair dryer. The unit is mounted on a drying hopper and hot air is circulated up through the material in the hopper, carrying surface moisture up and out of the top of the hopper.
Non-hygroscopic resins that utilize hygroscopic fillers will have higher moisture absorption, which can lead to moisture entrapped within the pellet itself. In these cases, a dehumidifying dryer should be used. Dehumidifying dryers are like a hot air dryer, with the exception that the air is dried using a desiccant bed before it is heated and circulated through the material in the hopper. This type of dryer removes surface moisture more effectively than hot air dryers.
Resins that absorb water within the pellets themselves, e.g., hygroscopic resins, require more sophisticated dryers, such as dehumidifying dryers. Two popular types of dehumidifying dryers include the twin tower desiccant or dual bed dryer and rotary wheel dryers. In twin tower desiccant dryers, one bed of desiccant supplies dry air to flow through the hopper, while simultaneously, a second bed of desiccant is being regenerated by forcing hot air through it. Rotary wheel dryers utilize a rotating wheel, which continuously places dry desiccant into service while regenerating moisture saturated desiccant in the same rotational cycle. This type of dryer results in a more consistent temperature and dew point for the hopper and can help to eliminate over drying.
There are additional dryer technologies that can be used for hygroscopic resins that do not use dehumidification. This absence of desiccant can save the molder not only the material expense, but also the time required to maintain and replace it. An example of one such technology is low pressure or vacuum dryers. These dryers accelerate the drying process by using a vacuum to lower the boiling point of water. This allows moisture to be rapidly extracted from the heated material. Typically, these dryers can dry materials in one-sixth of the time required by a desiccant dryer. The use of an appropriate dryer is not all that is needed to dry your resin. The maintenance and operation of the dryer selected are also critical for successful drying, Figure 3.

Figure 3. Guidelines for successful drying.
Moisture Analysis
Once the drying of material is underway, it is important that its moisture content is accurately measured during the drying process and before injection molding. Often, molders rely on a low dew point reading from the dryer to inform them whether they can begin processing. However, these readings measure the moisture of the air in the dryer, which does not guarantee that the resin is dry enough to process. The only way to know if the resin has been adequately dried is to directly measure the resin’s moisture content.
Gravimetric moisture analyzers are popular for determining the percentage of moisture in resin. In this technique, also referred to as “loss on drying,” a sample is placed inside of the equipment and its mass is compared before and after heating for a period of time to determine a percentage weight loss. This technique is very easy to conduct, but it does have some downsides. This technique lacks the ability to separate weight loss associated with water from other volatile ingredients, such as mold release, stabilizers, plasticizers, etc., which could lead to over drying the resin. This technique also produces less accurate measurements at the lower end of the detection limit, around 0.01%. If the material being dried is sensitive to low levels of moisture or requires less than 0.01% moisture before processing, an alternative analysis technique would be needed.
Another technique is Coulometric Karl Fischer Titration. This is an electrochemical technique, where a sample is introduced into an oven and its moisture is driven off and carried to the titration cell using a dry, inert gas. Once in the cell, a reagent reacts with water and converts it into a non-conductive chemical. The amount of electrical current required to convert the water is the determinant of the amount of moisture present. This technique is extremely accurate for moisture content levels below 1% and it can measure moisture content as low as 0.0001%. It provides results specific to the amount of water present and is not influenced by the presence of other volatile ingredients within the material. The downside is that this technique utilizes expensive chemicals and calibration standards. It also takes qualified personnel to operate and maintain this equipment. Since reagents will change over time they will need to be frequently replaced and due to the sensitivity of the equipment, it will require regular calibration.
Sensor-based moisture analyzers are available to determine the moisture content of resins both offline and online. In the case of offline analysis equipment, a sample is placed into an instrument oven, which heats the sample. A dry carrier gas is used to transport the volatiles from the sample to a sensor whose output is processed to generate an accurate moisture measurement. Like Coulometric Karl Fischer Titration, this technique is extremely accurate for moisture content levels down to as low as 0.001%. It also provides results specific to the amount of water present. Unlike Coulometric Karl Fischer Titration, this technique does not use expensive chemicals and it is easy to operate once it has been installed. For online analysis, the instrument is installed directly onto a drying hopper where it continuously measures the moisture content of the resin. Online sensors can measure moisture content below 0.001% to +/- 0.005%. This is the most streamlined option for production because it continuously measures moisture content, and the setup technician will know immediately when the resin is ready for processing. Offline testing is less efficient as one needs to acquire a sample, transport it to the location of the analysis equipment and then wait for the analysis to complete. This offline test may need to be performed multiple times over the course of drying before the desired limit is reached. However, the cost to implement online sensor-based moisture analysis is relatively higher when compared to the other techniques since multiple instruments are needed to be installed on the various drying hoppers. If the budget exists, inline sensing is the best option. If not, just be sure to select equipment that operators can run and that meet the needs of materials used within the facility.
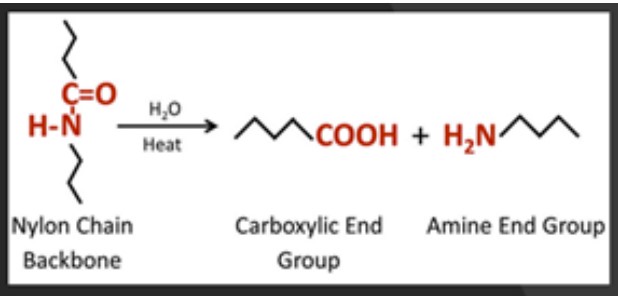
Figure 4. Hydrolysis of a nylon polymer chain.
Effects of Hydrolysis
Hydrolysis is defined as any chemical reaction that breaks one or more chemical bonds. Unlike water absorption itself, this hydrolysis is non-reversible. For hygroscopic polymers, hydrolysis or breaking of bonds occurs in the presence of absorbed water elevated temperatures, Figure 4. Hydrolysis will essentially act to reduce the molecular weight of the polymer, through depolymerization. Aside from the chemical composition of the polymer, molecular weight is one of the most significant determining factors of polymer properties. As molecular weight decreases so does mechanical performance, chemical resistance, as well as the viscosity of the polymer, Figure 5.
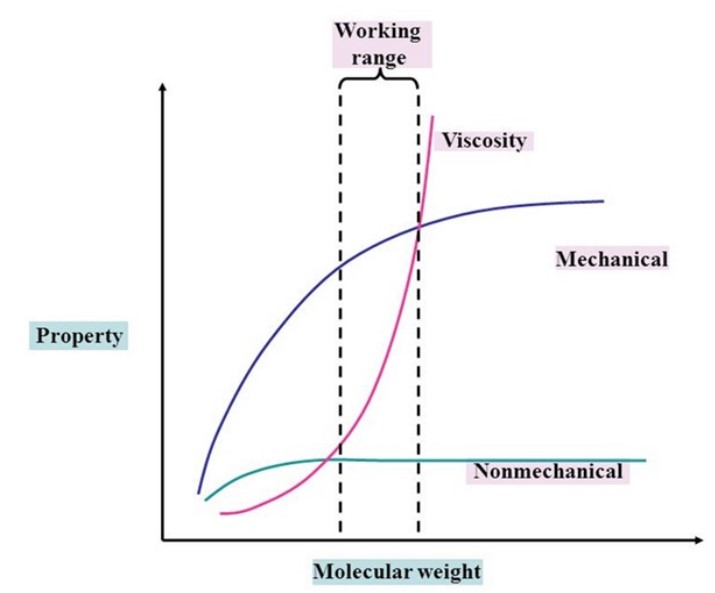
Figure 5. Effects of molecular weight on polymer performance. (Reference: International Journal of Hydrology)
Mechanical performance includes tensile properties, impact resistance, creep and fatigue resistance. Regarding tensile properties, at lower molecular weights, the polymer chains are loosely bonded by weak van der Waals forces and the chains can move easily, and therefore, resulting in lower strength, modulus, and elongation at break values. In the case of higher molecular weights, the chains become larger and hence are entangled, giving strength and ductility to the polymer.
The molecular weight also has an impact on material toughness. At higher molecular weights, the impact resistance of a polymer material increases. This is due to the higher degree of entanglement, which means that in order to rupture, more polymer bonds need to be broken. Therefore, a higher molecular weight polymer can absorb more energy before failing. Molecular weight will also affect the temperature at which, polymer behavior will transition from ductile to brittle. When molecular weight decreases the ductile-to-brittle transition temperature increases. For some materials this could mean altogether losing its ductile behavior at subambient temperatures.
Nonmechanical performance includes chemical resistance which in general, is dependent on molecular weight. At higher molecular weight, a polymer will demonstrate improved chemical resistance, especially in terms of environmental stress cracking (ESC). Conversely, at a lower molecular weight, chemical resistance will deteriorate. For many materials when melt flow rate (MFR) increases, aka molecular weight decreases, the time to ESC failure decreases, Table 2.
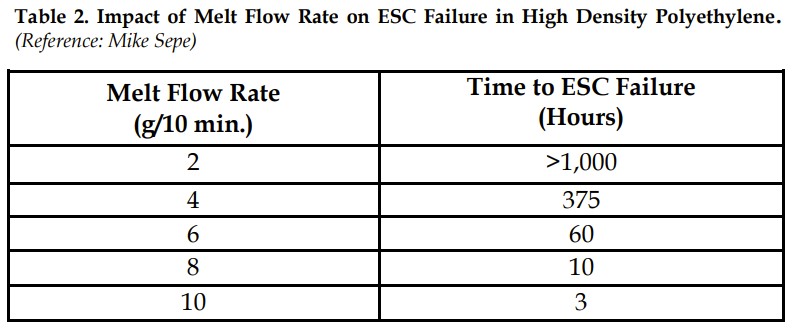
Table 2. Impact of Melt Flow Rate on ESC Failure in High Density Polyethylene. (Reference: Mike Sepe)
The changes in viscosity that occur in conjunction with changes in molecular weight will have a significant effect on processing. In injection molding, processing parameters are established for specific machine, mold, and resin combinations. Injection molders expect that resins will repeatedly behave in a known way during processing. In the case of improperly dried resins, as soon as hydrolysis begins and polymer chains start to shorten, the melt viscosity is no longer stable and will continuously decrease. The influence of these non-optimal processing conditions on molded parts could include overpacking, flash, splay and voiding. The processing of hygroscopic resins that have not been adequately dried will result in hydrolysis of the polymer, which has a detrimental impact on material performance. Molding of both hygroscopic and non-hygroscopic resins without appropriate drying, can result in part defects such as voiding and blistering as well as an undesirable appearance. Proper drying includes using the right drying technology as well as dryer parameters to ensure the resin is dried below the maximum moisture content prior to processing. Finally, it is critical to verify that the resin has been adequately dried by measuring its moisture content directly by using the appropriate moisture analyzer technology.
