Nylon, technically categorized as a polyamide (PA), is far from a singular material. Instead, it represents a diverse and sophisticated family of resins whose versatility has made it a cornerstone of modern engineering. Since Wallace Carothers first synthesized nylon at DuPont in the 1930s, the material has evolved into one of the most widely used thermoplastics in the world. Yet, as any engineer who has worked with polyamides knows, their greatest strength—their unique chemistry—is also the source of their most significant design challenge: water absorption.
The Influence of Molecular Structure
The performance of a polyamide is not a “one-size-fits-all” metric. Rather, it is dictated by its molecular architecture. Specifically, the ratio of paraffinic (methylene) carbon atoms to the polar amide functional groups determines the material’s identity.
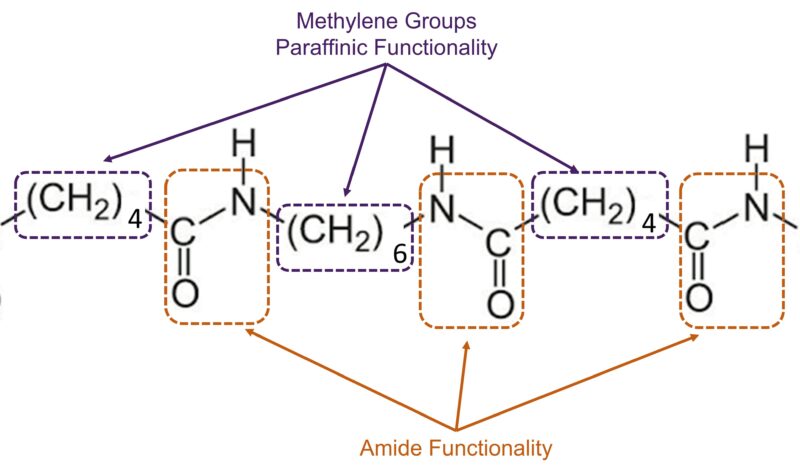
This structural relationship acts as the “DNA” of the polymer, influencing every critical performance metric, including:
- Mechanical properties: Such as tensile strength, stiffness, and ductility.
- Thermal characteristics: Including melting points and glass transition temperatures.
- Environmental resistance: The ability to withstand chemical attack and long-term degradation.
- Dimensional stability: The degree to which a part changes shape when exposed to the environment.
Water Absorption: The Great Plasticizer
Water absorption is perhaps the most critical variable to consider when designing with polyamides. In many polymers, moisture is a surface-level concern; in nylons, it is a fundamental property-altering event. Because the amide groups in nylon are polar, they have a natural affinity for water molecules.
When a nylon part is exposed to moisture—whether through atmospheric humidity or direct immersion—water molecules penetrate the polymer matrix and interfere with the intermolecular hydrogen bonding between chains. This process is known as plasticization.
The engineering consequences of this absorption are profound. As moisture levels increase:
- Strength and Modulus Decrease: The material becomes more flexible and less rigid.
- Impact Resistance Increases: The “softened” polymer chains can often absorb more energy before fracturing, making the material tougher but less dimensionally stable.
- Glass Transition Shifts: The temperature at which the material transitions from a rigid to a rubbery state can drop significantly, sometimes falling below the operating temperature of the application.
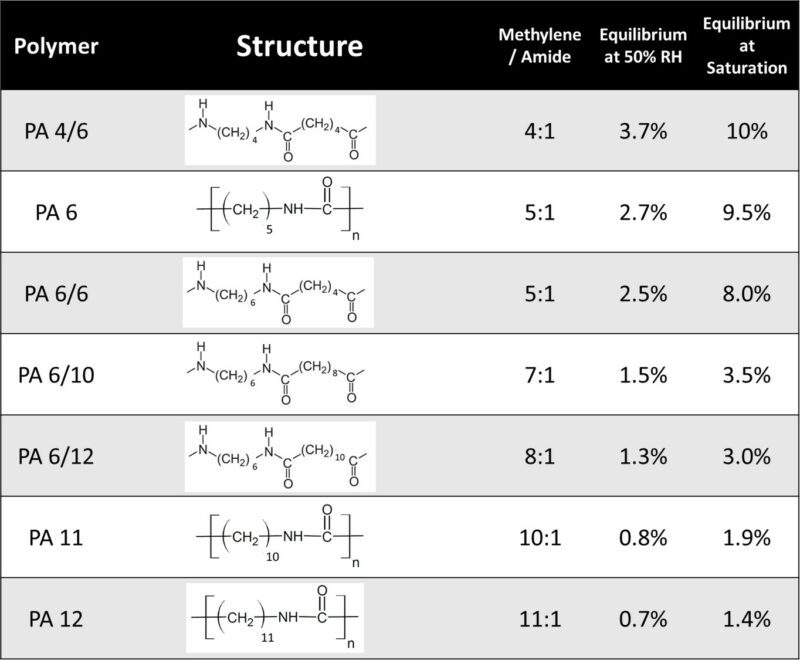
The Paraffinic Ratio: Predicting Performance
The hygroscopic nature of nylon is not a mystery; it is a direct function of its chemical nomenclature. In short-chain polyamides like Nylon 6, the high density of polar amide groups creates a high concentration of potential bonding sites for water molecules. In contrast, ‘long-chain’ polyamides such as Nylon 11 or Nylon 12 feature an increased ratio of non-polar methylene (paraffinic) groups. This shift in molecular architecture effectively shields the polymer from moisture, ensuring superior dimensional stability and mechanical property retention even in saturated environments.
Designing for Durability: The Madison Group Advantage
Understanding the water absorption of nylon is more than just a laboratory exercise; it is a prerequisite for avoiding catastrophic field failures. Because polyamides are so sensitive to their environment, selecting the wrong grade or failing to account for moisture-driven dimensional changes can lead to cracking, warping, or loss of critical mechanical margins.
Don’t wait for a field failure to discover that your material selection was a mismatch for the environment. Whether you are navigating the complexities of long-chain aliphatic nylons or high-performance PPAs, our team provides the technical clarity needed to move from design to production with total confidence.
Learn more about the performance of nylons here: Nylon in the Real World: Structure, Moisture, and Durability
